Cell Explorer™ Live Cell Labeling Kit
Green Fluorescence with 405 nm Excitation
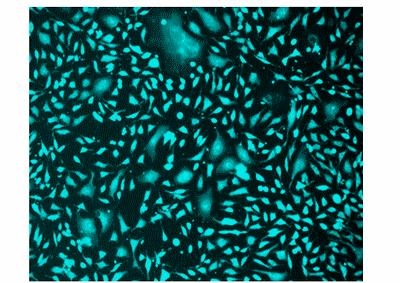
Our Cell Explorer™ fluorescence imaging kits are a set of tools for labeling cells for fluorescence microscopic investigations of cellular functions. The effective labeling of cells provides a powerful method for studying cellular events in a spatial and temporal context. This particular kit is designed to uniformly label live cells for the flow cytometric analysis of live cells with the violet laser (405 nm excitation). The kit uses a proprietary dye that gets enhanced fluorescence upon entering into live cells. The dye is a hydrophobic compound that easily permeates intact live cells. The hydrolysis of the weakly fluorescent substrate by intracellular esterases generates a strongly fluorescent hydrophilic product that is well-retained in the cell cytoplasm. It can be readily adapted for flow cytometry applications. The fluorescent dye used in the kit is well excited with the violet laser (405 nm excitation) to fluorescence at ~510 nm. The kit provides all the essential components with an optimized cell-labeling protocol. It is useful for a variety of studies, including cell adhesion, chemotaxis, multidrug resistance, cell viability, apoptosis and cytotoxicity.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22615 | 200 Tests | Price |
Spectral properties
| Excitation (nm) | 420 |
| Emission (nm) | 505 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 405 nm laser |
| Emission | 525/40 nm filter |
| Instrument specification(s) | AmCyan channel |
| Fluorescence microscope | |
| Excitation | Violet filter set |
| Emission | Violet filter set |
| Recommended plate | Black wall/clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 13, 2026

