Cell Meter™ Apoptotic and Necrotic Multiplexing Detection Kit II
Triple Fluorescence Colors
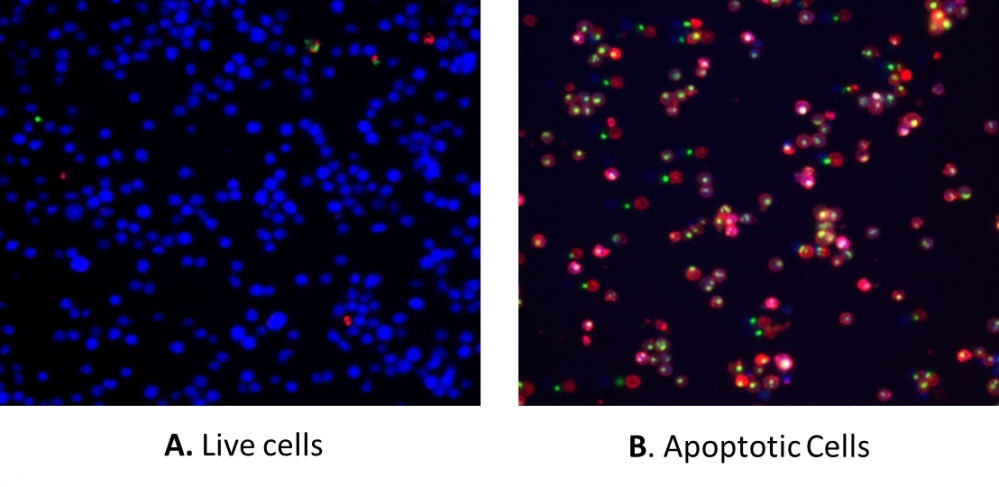
Our Cell Meter™ assay kits are a set of tools for monitoring cell viability. There are a variety of parameters that can be used. This particular kit is designed to simultaneously monitor apoptotic, necrotic, and healthy cells. Apoptosis is described as an active, programmed process of autonomous cellular dismantling that avoids eliciting inflammation. In apoptosis, phosphatidylserine (PS) is transferred to the outer leaflet of the plasma membrane. As a universal indicator of the initial/intermediate stages of cell apoptosis, the appearance of phosphatidylserine on the cell surface can be detected before morphological changes are observed. The PS sensor used in this kit has red fluorescence upon binding to membrane PS. Necrosis has been characterized as passive, accidental cell death resulting from environmental perturbations with the uncontrolled release of inflammatory cellular contents. Loss of plasma membrane integrity, as demonstrated by the ability of a membrane-impermeable DNA Nuclear Green™ DCS1 (Ex/Em = 490/525 nm) to label the nucleus, represents a straightforward approach to demonstrate late-stage apoptosis and necrosis. In addition, this kit also provides a live cell cytoplasm labeling dye, CytoCalcein™ Violet 450 (Ex/Em = 405/450 nm), for labeling living cell cytoplasm. This kit is optimized to simultaneously detect cell apoptosis (red and/or green), necrosis (green), and healthy cells (blue) with a flow cytometer or fluorescence microscope.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22843 | 100 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 405 nm, 488 nm, 633 nm laser |
| Emission | 450/40 nm, 530/30 nm, 660/20 nm filter |
| Instrument specification(s) | Pacific Blue, FITC, APC channel |
| Fluorescence microscope | |
| Excitation | DAPI, FITC, Cy5 filter sets |
| Emission | DAPI, FITC, Cy5 filter sets |
| Recommended plate | Black wall/clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 19, 2026
