Cell Meter™ Direct Cell Proliferation Assay Kit
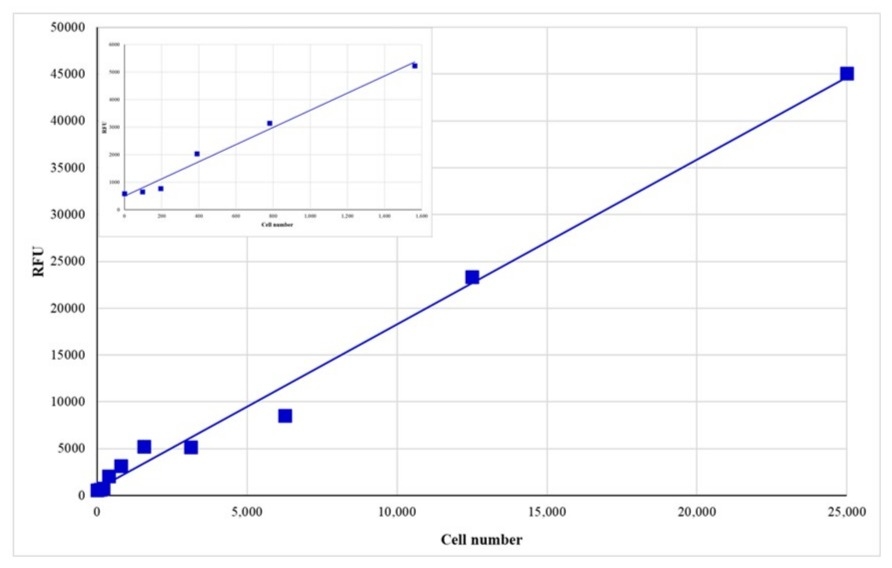
Cell Meter™ Direct Cell Proliferation Assay Kit is a DNA-based fluorescence assay optimized for high-throughput assessment of cell proliferation and cytotoxicity.
- Non-metabolic readout: Measures cell proliferation independent of metabolic activity for consistent results across conditions
- Streamlined workflow: Simple format eliminates the need for washes, lysis, or temperature equilibration
- Applications: Ideal for drug screening, cytotoxicity assays, and live-cell quantification across a wide dynamic range
- Comparable alternative: Offers an easy format similar to Thermo Fisher’s CyQUANT® Direct Cell Proliferation Assay

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22506 | 200 Tests | Price |
Spectral properties
| Excitation (nm) | 503 |
| Emission (nm) | 527 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 490 nm |
| Emission | 525 nm |
| Cutoff | 515 nm |
| Recommended plate | Black wall/Clear bottom plates |
| Instrument specification(s) | Bottom read mode |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 3, 2026

