Cell Meter™ Fixed Cell and Tissue TUNEL Apoptosis Assay Kit
Blue Fluorescence
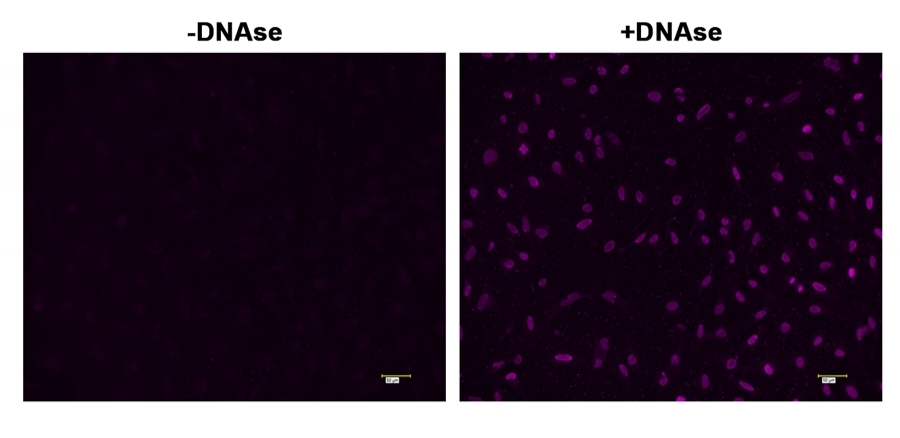
Cell Meter™ TUNEL apoptosis assay kit provides a robust tool for conveniently detecting apoptosis caused by DNA fragmentation. The assay is non-radioactive and rapid. The TUNEL assay uses the terminal deoxynucleotidyl transferase (TdT) to catalyze the incorporation of DEAC-dUTP at the free 3’-hydroxyl ends of the fragmented DNAs. The resulted DEAC-labeled DNAs are analyzed by fluorescence microscopy (AMC filter set). Its blue emission can be conveniently multiplexed with GFP labelled targets. Direct incorporation of fluorescent DEAC-labeled nucleotides significantly reduces the number of test steps. The kit is optimized to detect apoptosis in fixed cells and formalin-fixed, paraffin-embedded tissue sections.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22857 | 25 Tests | Price |
Spectral properties
| Correction factor (260 nm) | 0.14 |
| Correction factor (280 nm) | 0.12 |
| Excitation (nm) | 411 |
| Emission (nm) | 472 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Instrument settings
| Flow cytometer | |
| Excitation | 405 nm laser |
| Emission | 525/50 nm filter |
| Instrument specification(s) | Pacific Orange channel |
| Fluorescence microscope | |
| Excitation | Violet filter set |
| Emission | Violet filter set |
| Recommended plate | Black wall/clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on October 8, 2024

