Cell Meter™ Fluorimetric Intracellular Nitric Oxide (NO) Activity Assay Kit
Red Fluorescence Optimized for Flow Cytometry
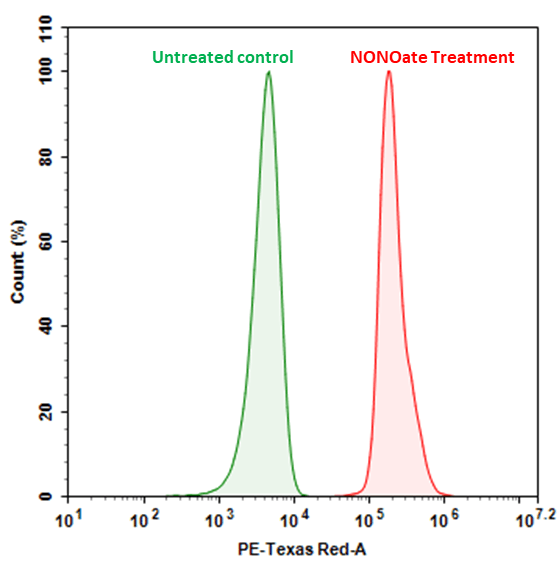
Nitric oxide (NO) is an important biological regulator involved in numbers of physiological and pathological processes. Altered NO production is implicated in various immunological, cardiovascular, neurodegenerative and inflammatory diseases. As a free radical, NO is rapidly oxidized and there is relatively low concentrations of NO existing in vivo. It has been challenging to detect and understand the role of NO in biological systems. Cell Meter™ Fluorimetric Intracellular Nitric Oxide Assay Kit provides a sensitive tool to monitor intracellular NO level in live cells. Nitrixyte™ probes are developed and used in our kit as an excellent replacement for DAF-2 for the detection and imaging of free NO in cells. Compared to the commonly used DAF-2 probe, Nitrixyte™ probes have better photostability and enhanced cell permeability. This particular kit uses Nitrixyte™ Red that can react with NO to generate a bright red fluorescent product that has spectral properties similar to Texas Red®. Nitrixyte™ Red can be readily loaded into live cells, and its fluorescence signal can be conveniently monitored using the filter set of Red. This kit is optimized for flow cytometry applications.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 16356 | 100 Tests | Price |
Spectral properties
| Excitation (nm) | 586 |
| Emission (nm) | 607 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 488 nm laser |
| Emission | 610/20 nm filter |
| Instrument specification(s) | Texas Red channel |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 27, 2026

