Cell Meter™ Fluorimetric Intracellular pH Assay Kit
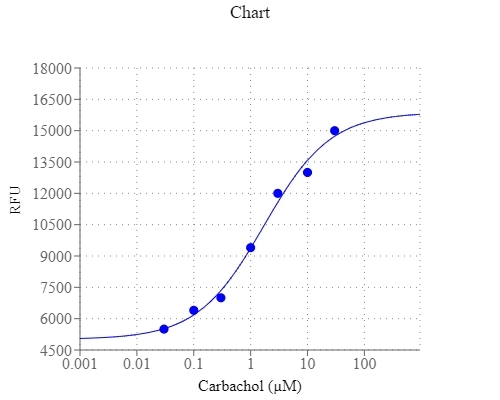
Intracellular pH change are implicated in diverse physiological and pathological processes, including cell proliferation, apoptosis, fertilization, malignancy, multidrug resistance, ion transport, lysosomal storage disorders and Alzheimer's disease. The Cell Meter™ Fluorimetric Intracellular pH Assay Kit utilizes AAT Bioquest's proprietary fluorescent indicator for measuring the relative intracellular pH changes. It is a homogeneous, kinetic, live-cell fluorescent assay that utilizes either a standard procedure or acid-load procedure. The standard protocol is designed for measuring the therapeutic targets of interest with a decrease in intracellular pH upon treatment. The 'Acid-Load' procedure is designed to measure the increase of intracellular pH associated with changes in cellular metabolism due to GPCR activation or growth factor activity. With the 'Acid-Load' procedure ammonium chloride solution is added after the fluorescent pH dye is loaded into cells in a minimum volume. This 'acid-loading' step is followed by the addition of agonist in a relatively large volume (~4X) of buffer. The sudden volume change initiates an efflux of ammonia (NH3) from the cells causing a rapid decrease in intracellular pH, and thus a decrease in fluorescence signal. The effect of agonist on the subsequent recovery of intracellular pH is measured by the relative fluorescence signal increase.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 21180 | 1000 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 490 nm |
| Emission | 535 nm |
| Cutoff | 515 nm |
| Recommended plate | Black well/Clear bottom |
| Other instruments | ArrayScan, FDSS, FLIPR, FlexStation, IN Cell Analyzer, NOVOStar, ViewLux |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 24, 2026
