Cell Meter™ Intracellular Fluorimetric Hydrogen Peroxide Assay Kit
Blue Fluorescence
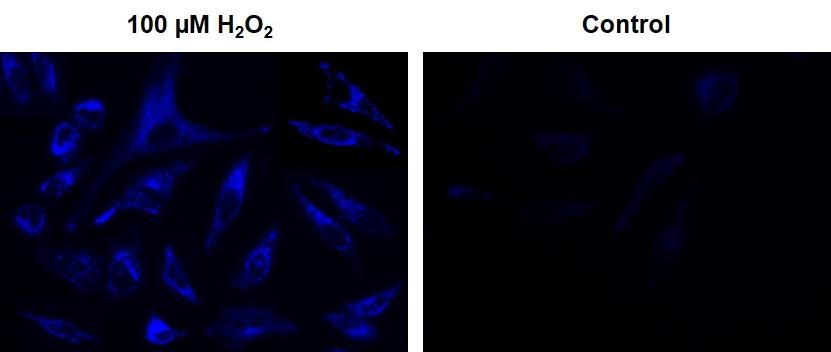
Hydrogen peroxide is a reactive oxygen metabolic by-product that serves as a key regulator for a number of oxidative stress-related states. It is involved in many biological events that are linked to asthma, atherosclerosis, diabetic vasculopathy, osteoporosis, a number of neurodegenerative diseases and Down's syndrome. The measurement of this reactive species is helpful for determining how oxidative stress modulates various intracellular pathways. This Cell Meter™ Intracellular Fluorimetric Hydrogen Peroxide Assay Kit uses our unique OxiVision™ Blue peroxide sensor to quantify hydrogen peroxide in live cells. OxiVision™ Blue peroxide sensor is cell-permeable, and generates blue fluorescence when it reacts with hydrogen peroxide. This kit provides a sensitive tool to monitor hydrogen peroxide level in living cells, and it is optimized to be used for fluorescence microscopy.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 11504 | 100 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Instrument settings
| Fluorescence microscope | |
| Excitation | DAPI filter |
| Emission | DAPI filter |
| Recommended plate | Black wall/clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on March 6, 2026
