Cell Meter™ Mitochondrion Membrane Potential Assay Kit
Orange Fluorescence Optimized for Flow Cytometry
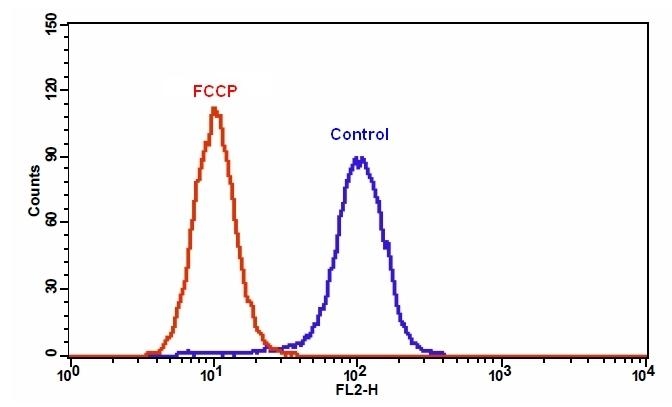
This particular kit is designed to detect cell apoptosis by measuring the loss of the mitochondrial membrane potential(MMP). The collapse of mitochondrial membrane potential coincides with the opening of the mitochondrial permeability transition pores, leading to the release of cytochrome C into the cytosol, which in turn triggers other downstream events in the apoptotic cascade. Our Cell Meter™ Orange Mitochondrial Membrane Potential Assay Kit provides all the essential components with an optimized assay method. This fluorimetric assay uses our proprietary cationic MitoLite™ Orange for the detection of apoptosis in cells with the loss of mitochondrial membrane potential. In normal cells, the red fluorescence intensity is increased when MitoLite™ Orange is accumulated in the mitochondria. However, in apoptotic cells, the fluorescence intensity of MitoLite™ Orange is decreased following the collapse of MMP. Cells stained with MitoLite™ Orange can be visualized with a flow cytometer at 488 nm excitation with red emission (FL2 channel). The kit can be used together with other reagents, such as Cell Meter™ Phosphatidylserine Apoptosis Assay Kit (22835) for multi-parametric study of cell vitality and apoptosis. The kit is optimized for screening apoptosis activators and inhibitors with a flow cytometer.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22804 | 100 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 488 nm or 532 nm laser |
| Emission | 575/26 nm filter |
| Instrument specification(s) | PE channel |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 20, 2026
