Cell Meter™ Multiplexing Live, Apoptotic and Necrotic Cell Detection Kit III
Triple Fluorescence Colors
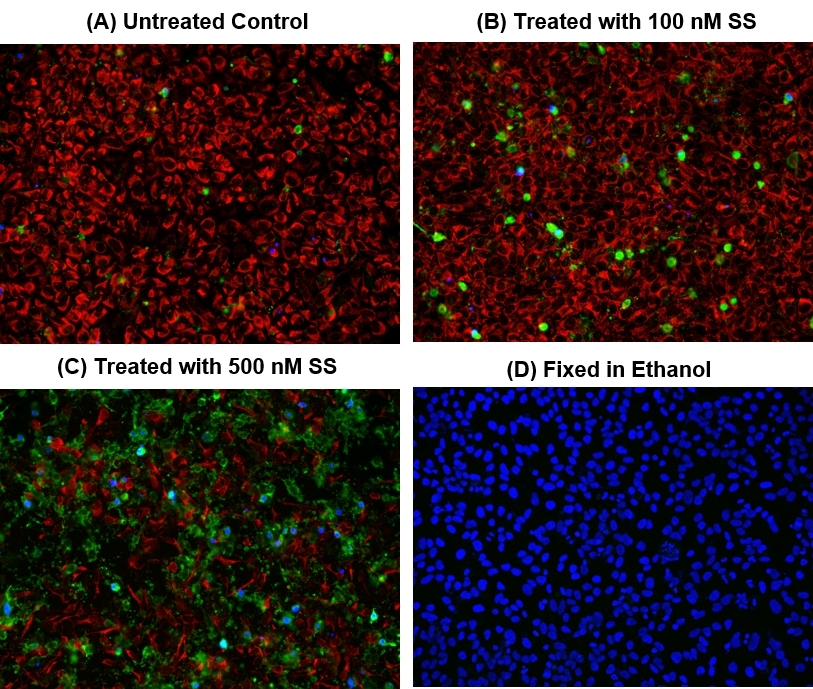
Our Cell Meter™ assay kits are a set of tools for monitoring cell viability. There are a variety of parameters that can be used. This particular kit is designed to simultaneously monitor apoptotic, necrotic and healthy cells. Apoptosis is an active, programmed process of autonomous cellular dismantling that avoids eliciting inflammation. In apoptosis, phosphatidylserine (PS) is transferred to the outer leaflet of the plasma membrane. As a universal indicator of the initial/intermediate stages of cell apoptosis, the appearance of phosphatidylserine on the cell surface can be detected before morphological changes are observed. The PS sensor Annexin V-iFluor® 488 conjugate has green fluorescence upon binding to membrane PS. Necrosis has been characterized as passive, accidental cell death resulting from environmental perturbations with uncontrolled release of inflammatory cellular contents. Loss of plasma membrane integrity, as demonstrated by the ability of a membrane-impermeable Nuclear Blue™ DCS1 (Ex/Em = 350/461 nm) to label the nucleus, represents a straightforward approach to demonstrate late stage of apoptosis and necrosis. In addition, this kit also provides a live cell labeling dye, Cellbrite™ Red (Ex/Em = 613/631 nm), for labeling non-apoptotic healthy cells. This kit is optimized to simultaneously detect cell apoptosis (green), necrosis (blue and/or green) and healthy cells (red) with a fluorescence microscope.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22846 | 200 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microscope | |
| Excitation | 494 nm (apoptosis) / 350 nm (necrosis) / 613 nm (live) |
| Emission | 520 nm (apoptosis) / 461 nm (necrosis) / 631 nm (live) |
| Recommended plate | Black wall/clear bottom |
| Instrument specification(s) | FITC filter (apoptosis) / DAPI filter (necrosis) / Cy5 filter (live) |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 19, 2026
