Cell Navigator® Fluorimetric Lipid Droplet Assay Kit
Blue Fluorescence
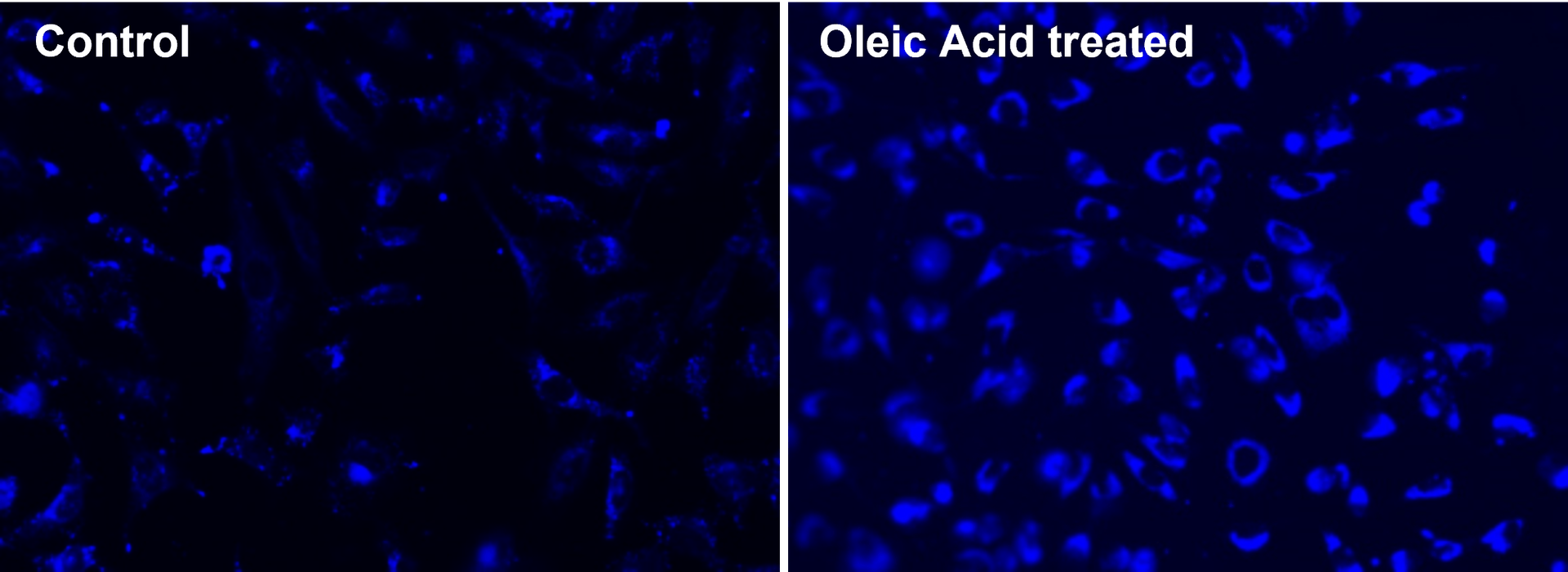
Cell Navigator® Fluorimetric Lipid Droplet Assay Kit is ideal for detecting and analyzing lipid droplets in diverse research applications.
- Minimal background: High specificity for lipid droplets with minimal background noise
- Versatile applications: Compatible with fluorescence microscopy, flow cytometry, and fluorescence microplate readers
- Comparable alternative: Provides lower background and higher signal specificity comparable to LipidTOX™ neutral lipid stains from Thermo Fisher
- Non-toxic: Suitable for live cell imaging, offering a safe method to study lipid droplets in real time

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22731 | 200 Tests | Price |
Physical properties
| Solvent | DMSO |
Spectral properties
| Excitation (nm) | 336 |
| Emission (nm) | 443 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microscope | |
| Excitation | DAPI filter set |
| Emission | DAPI filter set |
| Recommended plate | Black wall/clear bottom |
| Fluorescence microplate reader | |
| Excitation | 350 nm |
| Emission | 450 nm |
| Cutoff | 420 nm |
| Recommended plate | Black wall/clear bottom |
| Instrument specification(s) | Bottom read mode |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 29, 2026

