Cell Navigator® Lysosome Staining Kit
Green Fluorescence with 405 nm Excitation
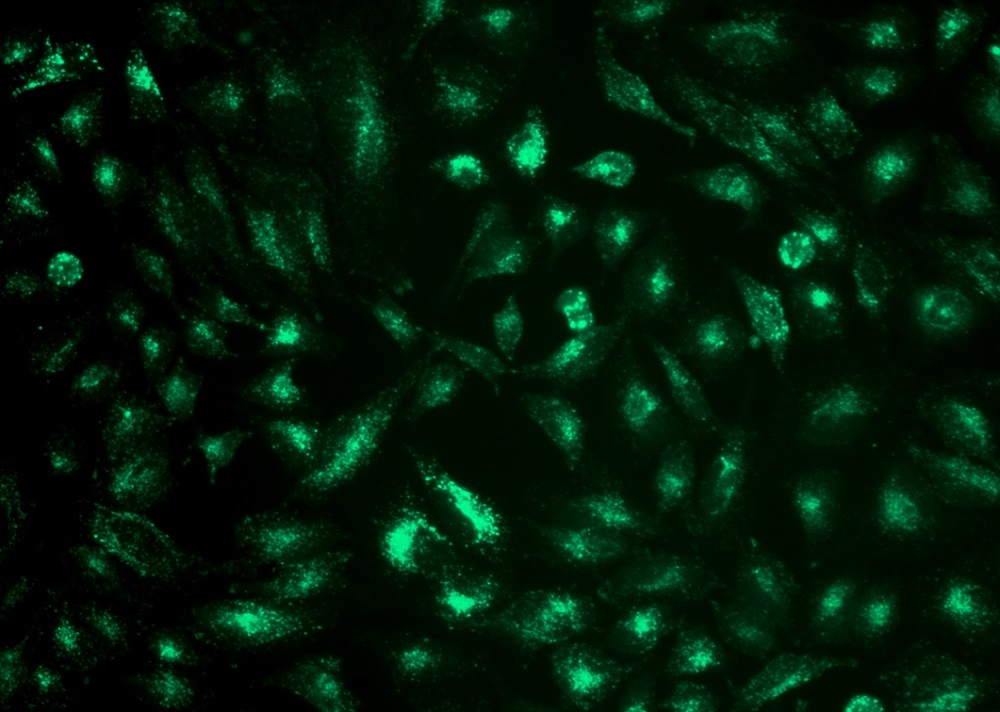
Our Cell Navigator® fluorescence imaging kits are a set of fluorescence imaging tools for labeling sub-cellular organelles such as membranes, lysosomes, mitochondria and nuclei etc. The selective labeling of live cell compartments provides a powerful method for studying cellular events in a spatial and temporal context. This particular kit is designed to label lysosomes of live cells in green fluorescence. The kit uses a proprietary lysotropic dye that selectively accumulates in lysosomes probably vial the lysosome pH gradient. The lysotropic indicator is a hydrophobic compound that easily permeates intact live cells, and trapped in lysosomes after it gets into cells. Its fluorescence is significantly enhanced upon entering lysosomes. This key feature significantly increases its selectivity for lysosomes. The labeling protocol is robust, requiring minimal hands-on time. It can be readily adapted for a wide variety of fluorescence platforms such as microplate assays, immunocytochemistry and flow cytometry. It is useful for a variety of studies, including cell adhesion, chemotaxis, multidrug resistance, cell viability, apoptosis and cytotoxicity. The kit provides all the essential components with an optimized cell-labeling protocol. It is suitable for proliferating and non-proliferating cells, and can be used for both suspension and adherent cells. This kit is optimized for flow cytometers equiped with viloet laser excitation @ 405 nm and emission around 500 nm.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22651 | 500 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microscope | |
| Excitation | 405 nm |
| Emission | 480 nm |
| Recommended plate | Black wall/clear bottom |
| Instrument specification(s) | Violet filter set |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 30, 2026
