CytoTell® Green
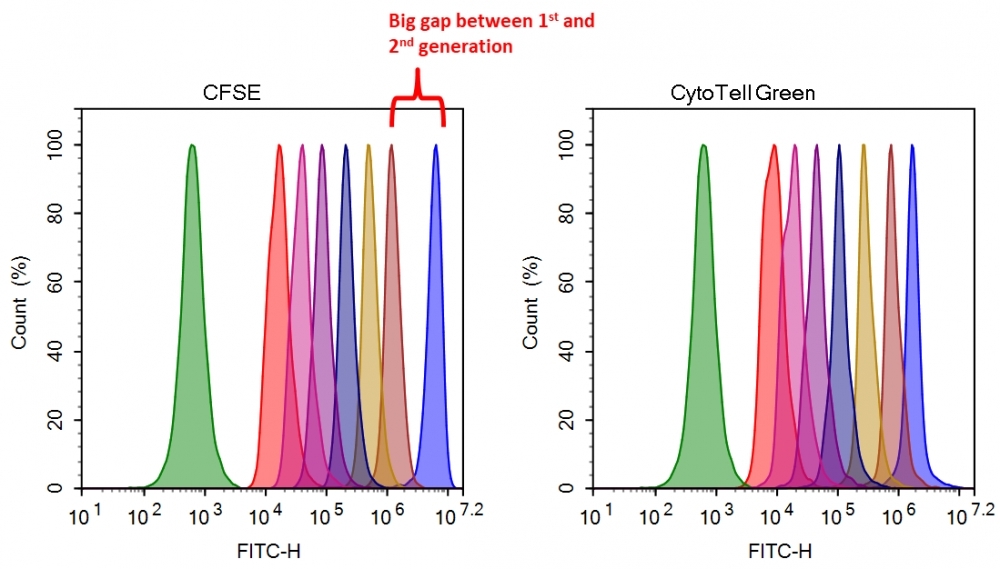
Flow cytometry combined with fluorescence staining is a powerful tool to analyze heterogeneous cell populations. Among all the existing fluorescent dyes CFSE is the preferred cell proliferation indicator that is widely used for live cell analysis. However, there are a few severe problems associated with the use of CFSE for monitoring cell proliferation. 1). CFSE is highly toxic to cells. CFSE indiscriminately reacts with all amino groups, thus changes many critical intracellular protein functions (such as cell membrane GPCRs); 2). CFSE has slow response and is inconvenient to use. The CFSE fluorescence intensity of the 2nd generation cells is decreased more than 10 fold from the 1st generation. You would have to wait for another generation to start the cell proliferation analysis. 3). Medium removal is required. You would have to remove medium for cell analysis with a flow cytometer since CFSE reacts with medium components. CytoTell® Green is developed to eliminate these limitations. It has distinct advantages over CFSE. 1). CytoTell® Green has very minimal cytotoxicity compared to CFSE. It is well retained in cells since the probe is designed to minimize the MDR interaction that generally pumps many cell stains out cells; 2). CytoTell® Green exhibits much faster response and is more convenient to use than CFSE. There is no fluorescence intensity gap between 1st and 2nd generation of cells. As cells divide, CytoTell® Green is distributed equally between daughter cells that can be measured as successive halving of the fluorescence intensity of the dye; 3). There is no need to remove medium since CytoTell® Green does not react with medium components; 4). CytoTell® Green is more sensitive than CFSE. Up to 9 generations may be visualized; 5). CytoTell® Green is much more stable than CFSE. CytoTell® Green stock solution can be stored at room temperature for a few days. CytoTell® Green can also be used for long term tracking of labeled cells. Analysis using two-parameter plots may provide better resolution of each generation, especially between undivided cells and the first generation. Cells labeled with CytoTell® Green may be fixed and permeabilized for analysis of intracellular targets using standard formaldehyde-containing fixatives and saponin-based permeabilization buffers. CytoTell® Green has a peak excitation of 510 nm and can be excited by the blue (488 nm) laser line, making it compatible with FITC filter set.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22253 | 500 Tests | Price | |
| 22254 | 2x500 Tests | Price |
Physical properties
| Molecular weight | 419.24 |
| Solvent | DMSO |
Spectral properties
| Excitation (nm) | 510 |
| Emission (nm) | 525 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 488 nm laser |
| Emission | 530/30 nm filter |
| Instrument specification(s) | FITC channel |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 20, 2026

