Droplite™ Green
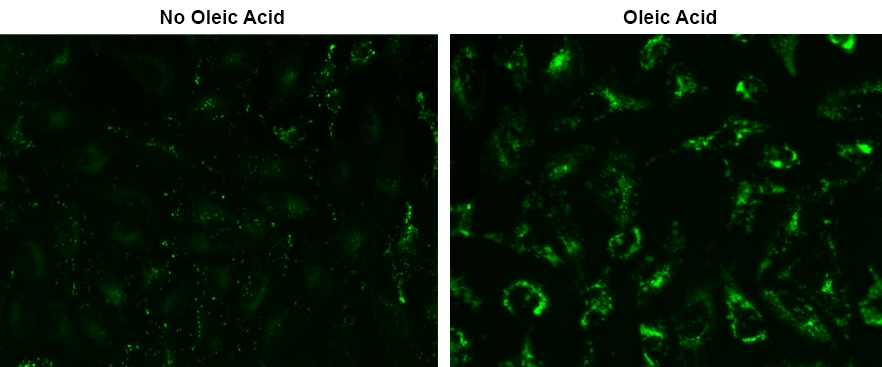
Lipid Droplets (LDs) are essential organelles responsible for storing neutral lipids, primarily consisting of triglycerides and cholesterol esters. Found in a variety of cell types, LDs play crucial roles in numerous biological processes, including metabolism, membrane biosynthesis, cell signaling, inflammation, and cancer-related mechanisms. Droplite™ Green, developed by AAT Bioquest, is a green fluorescent dye with an extremely high affinity for LDs and minimal cell toxicity. This probe has an excitation and emission maxima of 421 nm and 521 nm, respectively, and can be conveniently detected using fluorescence microscopy or an HCS reader. In cell culture experiments, Droplite™ Green demonstrates exceptional safety, even at high concentrations, ensuring reliable and accurate LD characterization without compromising cell health and viability.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22729 | 100 Tests | Price |
Physical properties
| Molecular weight | 432.37 |
| Solvent | DMSO |
Spectral properties
| Excitation (nm) | 421 |
| Emission (nm) | 521 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microscope | |
| Excitation | FITC filter set |
| Emission | FITC filter set |
| Recommended plate | Black wall/clear bottom |
| Instrument specification(s) | FITC filter set |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on March 3, 2026

