FluoroQuest™ Fluorescence Quantum Yield Determination Kit
Optimized for Bioconjugates
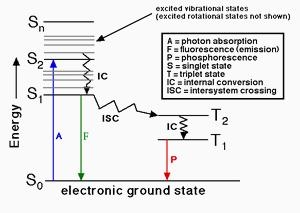
When a fluorophore absorbs a photon of light, an energetically excited state is formed. The fate of this species is varied, depending upon the exact nature of the fluorophore and its surroundings, but the end result is deactivation (loss of energy) and return to the ground state. The main deactivation processes which occur are fluorescence (loss of energy by emission of a photon), internal conversion and vibrational relaxation (non-radiative loss of energy as heat to the surroundings), and intersystem crossing to the triplet manifold and subsequent non-radiative deactivation. The fluorescence quantum yield is the ratio of photons absorbed to photons emitted through fluorescence. In other words the quantum yield gives the probability of the excited state being deactivated by fluorescence rather than by another, non-radiative mechanism. The kit provides all the essential components for fluorescence quantum yield determination for biological conjugates. It is optimized for determining the fluorescence quantum yields of fluorescent protein conjugates, peptides, nucleotides and nucleic acids.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 20 | 1 kit | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Instrument settings
| Spectrofluorometer | |
| Excitation | See Table 1 |
| Emission | See Table 1 |
| Fluorescence microplate reader | |
| Excitation | See Table 1 |
| Emission | See Table 1 |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 20, 2026
