Helixyte™ Fluorimetric RNA Quantification Kit
20-1000 ng Broad Range
Helixyte™ Fluorimetric RNA Quantification Kit is ideal for accurate, high-throughput RNA detection across diverse research applications.
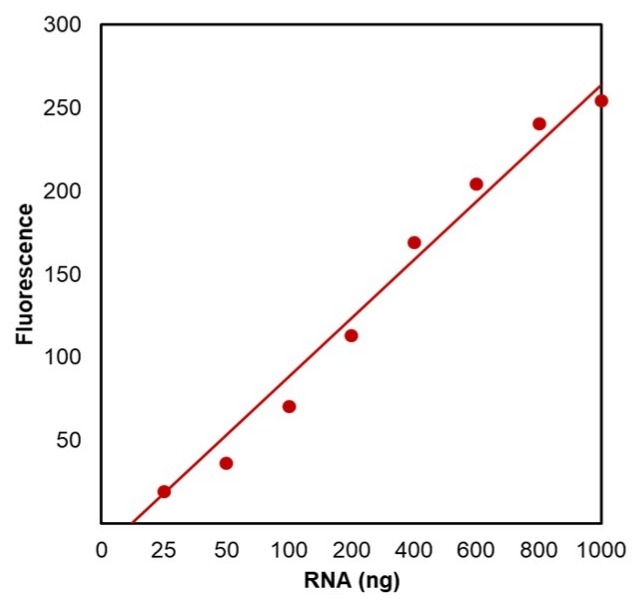
- Broad range detection: Accurately quantifies RNA in the 20–1000 ng range using a fluorescence-based assay
- High specificity: Selectively binds to RNA with minimal background from DNA, proteins, or other contaminants
- Applications: Suitable for RT-qPCR, RNA-seq, cDNA synthesis, and other RNA-based assays
- Comparable alternative: Offers a similar dynamic range to Qubit RNA Broad-Range Assay Kits while enabling use with standard fluorescence microplate readers

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 17698 | 500 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 640 nm |
| Emission | 675 nm |
| Cutoff | 665 nm |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on March 3, 2026
