iFluor® 460 Styramide
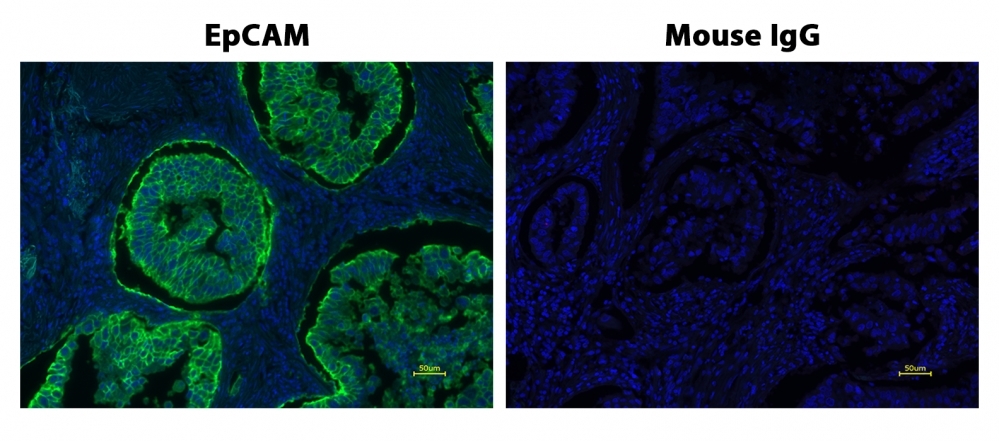
Power Styramide™ Signal Amplification (PSA™) system is one of the most sensitive methods that can detect extremely low-abundance targets in cells and tissues with improved fluorescence signal 10-50 times higher than the widely used tyramide (TSA) reagents. In combination with our superior iFluor® dyes that have higher florescence intensity, increased photostability and enhanced water solubility, the iFluor® dye-labeled Styramide™ conjugates can generate fluorescence signal with significantly higher precision and sensitivity (more than 100 times) than standard ICC/IF/IHC. Compared to tyramide reagents, the Styramide™ conjugates have ability to label the target at higher efficiency and thus generate significantly higher fluorescence signal. Styramide™ conjugates also allow significantly less consumption of primary antibody compared to standard directly conjugate method or tyramide amplification with the same level of sensitivity. iFluor® 460 Styramide is a new unique PSA™ reagent for multicolor application with our existing PSA™ and TSA reagents. It is optimized with the instruments equipped with the 460 nm laser or other equivalent light sources such as LEDs.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 44902 | 100 Slides | Price |
Physical properties
| Molecular weight | 975.27 |
| Solvent | DMSO |
Spectral properties
| Correction factor (260 nm) | 0.98 |
| Correction factor (280 nm) | 0.46 |
| Extinction coefficient (cm -1 M -1) | 80000 1 |
| Excitation (nm) | 468 |
| Emission (nm) | 493 |
| Quantum yield | ~0.8 1 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Instrument settings
| Fluorescence microscope | |
| Excitation | FITC filter set |
| Emission | FITC filter set |
| Recommended plate | Black wall/clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 24, 2026

