iFluor® 647 Tetrazine
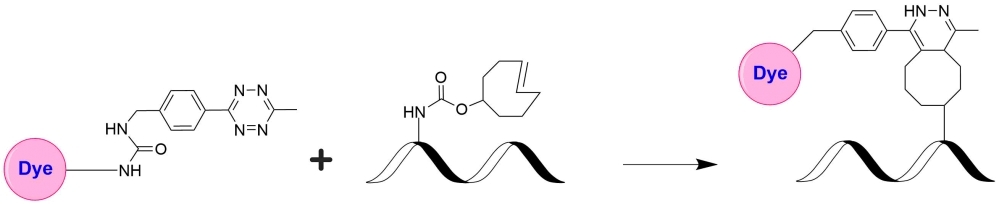
The tetrazine-trans-cyclooctene (TCO) ligation constitutes a non-toxic biomolecule labeling method of unparalleled speed. A tetrazine-functionalized molecule reacts with a TCO-functionalized molecule, forming a stable conjugate via a dihydropyrazine moiety. This has gained popularity due to its extremely fast kinetics. AAT Bioquest offers a group of tetrazine- and TCO-containing dyes for exploring various biological systems that can use this powerful click reaction. iFluor® 647 tetrazine can be readily used to label TCO-modified biological molecules for fluorescence imaging and other fluorescence-based biological applications. The conjugates prepared with iFluor® 647 dye have spectral properties almost identical to the popular Cy5 and Alexa Fluor® 647. In most cases, antibody conjugates prepared with iFluor® 647 tend to have a higher signal/background ratio than the spectrally similar dye conjugates, such as Cy5 and Alexa Fluor® 647 (Alexa Fluor® is the trademark of Invitrogen).

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 1019 | 1 mg | Price |
Physical properties
| Molecular weight | 1272.44 |
| Solvent | DMSO |
Spectral properties
| Correction factor (260 nm) | 0.03 |
| Correction factor (280 nm) | 0.03 |
| Correction factor (656 nm) | 0.0793 |
| Extinction coefficient (cm -1 M -1) | 250000 1 |
| Excitation (nm) | 656 |
| Emission (nm) | 670 |
| Quantum yield | 0.25 1 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 31, 2026

