iFluor® Ultra 750 maleimide
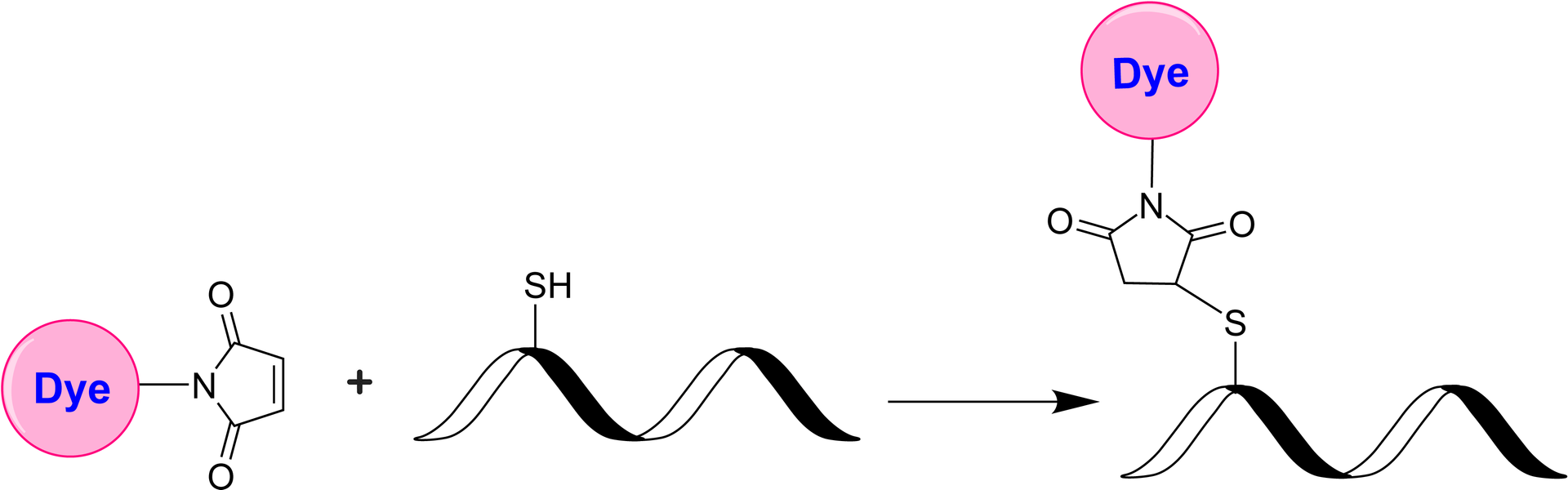
The iFluor® Ultra series represents an enhancement of our established iFluor® dyes, optimized for antibody labeling in fluorescence imaging and flow cytometry. Within this series, iFluor® Ultra 750 is a bright NIR fluorescent dye excitable by 633 nm to 685 nm laser lines, with a peak emission at 773 nm. Antibody conjugates with iFluor® Ultra 750 demonstrate superior brightness compared to those labeled with spectrally similar dyes such as Cy7, Dylight 755, IRDye750, and Alexa Fluor® 750 under the same conditions. Additionally, iFluor® Ultra 750 maintains stable fluorescence across a pH range of 4 to 10. The maleimide derivative of iFluor® Ultra 750 is widely used for conjugation to thiol groups on proteins, oligonucleotide thiophosphates, or low molecular weight ligands. Conjugates with iFluor® Ultra 750 demonstrate higher fluorescence intensity and greater photostability compared to those with other spectrally similar fluorophores, enhancing their utility for advanced fluorescence applications. Fluorescent dye-conjugated antibodies are crucial for protein identification in various applications, including fluorescent cell imaging, flow cytometry, western blotting, and immunohistochemistry. The advantages of these conjugates include increased sensitivity, multiplexing capabilities, and ease of use, facilitating complex biological studies.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 71683 | 1 mg | Price |
Physical properties
| Molecular weight | 1208.27 |
| Solvent | DMSO |
Spectral properties
| Absorbance (nm) | 750 |
| Correction factor (260 nm) | 0.04 |
| Correction factor (280 nm) | 0.05 |
| Correction factor (565 nm) | 0.021 |
| Correction factor (650 nm) | 0.149 |
| Extinction coefficient (cm -1 M -1) | 250000 1 |
| Excitation (nm) | 749 |
| Emission (nm) | 773 |
| Quantum yield | 0.32 1 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 23, 2026

