LAMP Green™
50X DMSO Solution
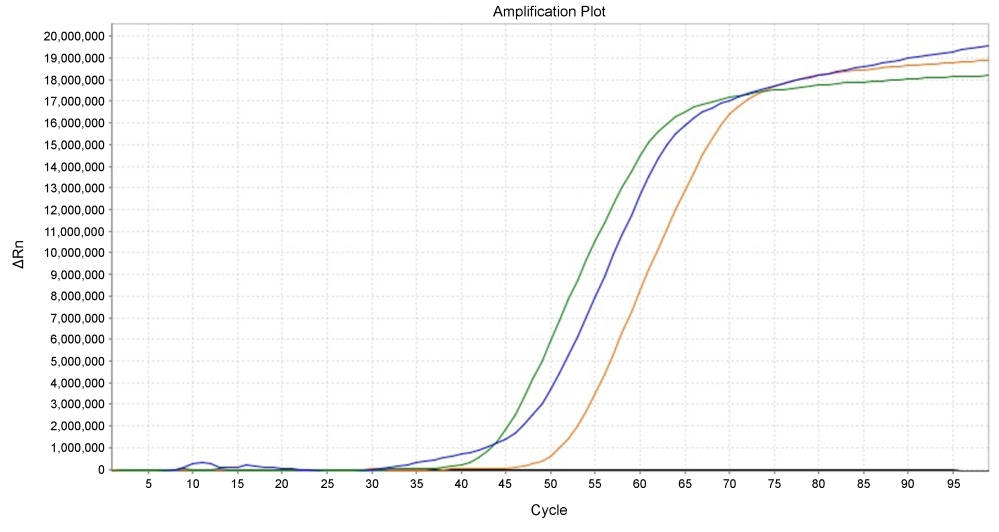
Isothermal amplification methods provide detection of nucleic acid target sequence in a streamlined, exponential manner. The existing dyes that successfully detect the LAMP reaction are mostly based on the end point method using the colorimetric analysis. LAMP Green™ enables real-time fluorescence measurement of a LAMP reaction. The dye can be detected using the SYBR or FAM channel on common real-time PCR instruments. LAMP Green™ is compatible with the agarose gel electrophoresis, making it possible to run on a gel and analyze it by gel imager.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 17555 | 100 ul | Price |
Physical properties
| Molecular weight | 578.73 |
| Solvent | DMSO |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 16, 2026
