Mag-520™ potassium salt
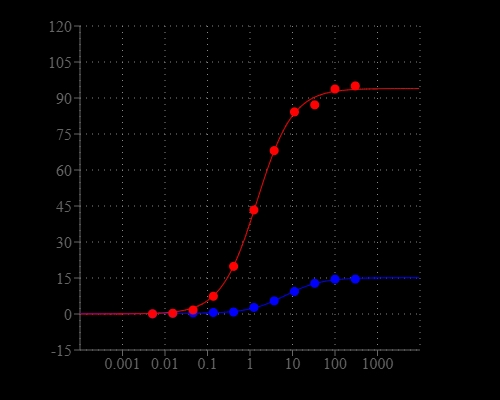
A vast majority of the existing magnesium ion indicators are based on tricarboxylate APTRA chelator derived from the popular tetracarboxylate BAPTA calcium ion chelator. They include mag-fura-2, mag-indo-1, mag-fluo-4 and mag-rhod-3. However, all of them have higher affinity for calcium than magnesium although they were designed for detecting magnesium ion. Mag-520™ is the first commercial magnesium indicator that has higher affinity for magnesium than calcium. Its significantly improved selectivity can be used for measuring magnesium ion with minimal interference from calcium ion compared to other commercial magnesium ion indicators such as the popular mag-fluo-4.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 20404 | 10x50 ug | Price |
Physical properties
| Molecular weight | 511.51 |
| Solvent | Water |
Spectral properties
| Extinction coefficient (cm -1 M -1) | 75000 1 |
| Excitation (nm) | 507 |
| Emission (nm) | 531 |
Storage, safety and handling
| Intended use | Research Use Only (RUO) |
| Storage | Freeze (< -15 °C); Minimize light exposure |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 24, 2026

