MycoLight™ Green JJ99
5 mM in DMSO
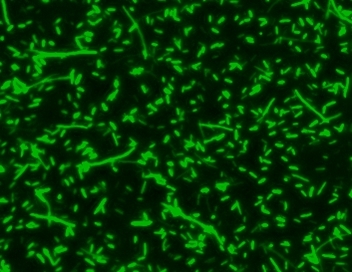
MycoLight™ Green JJ99 stain is an excellent green-fluorescent nuclear and chromosome counterstain that is permeant to both prokaryotic and eukaryotic cell membranes. MycoLight™ Green JJ99 stain has a high affinity for DNA and exhibits enhanced fluorescence upon binding with an excitation maximum close to the 488 nm argon laser line and fluorescence emission maximum at ≤500 nm. MycoLight™ Green JJ99 stain is particularly useful as a nuclear counterstain for bacterial assays since it stains both live and dead Gram-positive and Gram-negative bacteria. It is an excellent replacement for SYTO® 9 (SYTO® is the trademark of Invitrogen).

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 24001 | 100 ul | Price |
Physical properties
| Molecular weight | N/A |
| Solvent | DMSO |
Spectral properties
| Excitation (nm) | 482 |
| Emission (nm) | 512 |
Storage, safety and handling
| H-phrase | H301, H311, H331 |
| Hazard symbol | T |
| Intended use | Research Use Only (RUO) |
| R-phrase | R23, R24, R25 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 488 nm laser |
| Emission | 530/30 nm filter |
| Instrument specification(s) | FITC channel |
| Fluorescence microscope | |
| Excitation | FITC filter set |
| Emission | FITC filter set |
| Recommended plate | Black wall/clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 18, 2026

