MycoLight™ Red JJ94
2.5 mM in DMSO
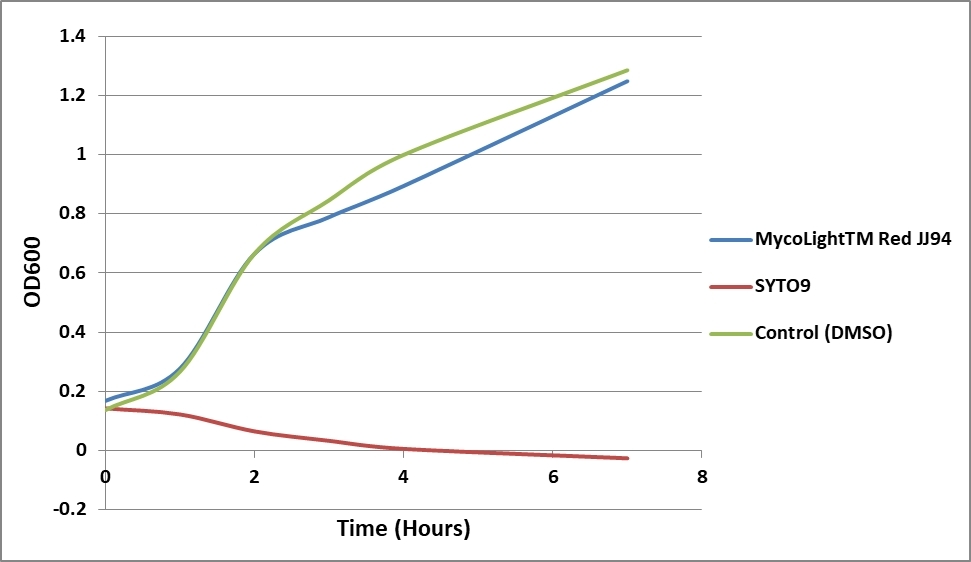
Green fluorescent protein (GFP) derivatives are widely used as fluorescent reporters to study biological processes at the cellular and molecular level, including labeling bacteria. Unfortunately, the GFP-based bacterial labeling is tedious, and several important organisms have remained difficult to modify for fluorescent protein expression. Complimentary to GFP-based fluorescence labeling of bacteria, SYTO-9 has been widely used for labeling bacteria in a more convenient mode, i.e., through a simple incubation. However, SYTO-9 is reported to significantly inhibit some bacterial growth. AAT Bioquest has developed MycoLight™ Red JJ94 that has minimal inhibition of bacterial growth compared to SYTO-9. In addition, MycoLight™ Red JJ94 can be used for multicolor detection of bacteria with the widely used GFP probes. It has an excitation and emission at ~637 and ~651 nm, respectively. This perfectly matches the He-laser excitation and Cy5 filter set that are commonly equipped in most fluorescence microscopes and flow cytometers. MycoLight™ Red JJ94 is a far-red fluorescent DNA-binding dye for straightforward live bacterial cell labeling, allowing subsequent infection studies. It has low intrinsic fluorescence, but upon binding DNA, its fluorescence emission intensity increases dramatically. MycoLight™ Red JJ94 readily stains live bacteria without compromising microbial growth. Under the same conditions, SYTO-9 inhibits bacterial growth substantially while MycoLight™ Red JJ94 is fully compatible with normal bacterial growth in liquid medium or on solid medium. The bacterial fluorescence labeling by MycoLight™ Red JJ94 is stable over several hours after shifting the bacteria into dye-free media.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 24006 | 100 uL | Price |
Physical properties
| Molecular weight | 738.75 |
| Solvent | DMSO |
Storage, safety and handling
| H-phrase | H301, H311, H331 |
| Hazard symbol | T |
| Intended use | Research Use Only (RUO) |
| R-phrase | R23, R24, R25 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 640 nm laser |
| Emission | 660/20 nm filter |
| Instrument specification(s) | APC channel |
| Fluorescence microscope | |
| Excitation | 630 nm |
| Emission | 660 nm |
| Recommended plate | Black wall/clear bottom |
| Instrument specification(s) | Cy5 filterset |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 25, 2026
