Nuclear Violet™ DCS1
5 mM DMSO Solution
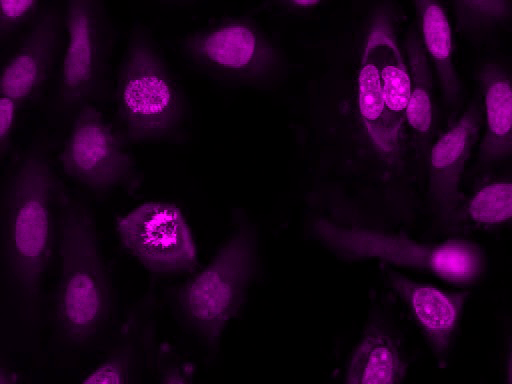
Our Nuclear Violet™ DCS1 is a fluorogenic, DNA-selective and live cell-impermeant dye for analyzing DNA content in dead or fixed cells. The Nuclear Violet™ DCS1 has its blue fluorescence significantly enhanced upon binding to DNA. It can be used in fluorescence imaging, microplate and flow cytometry applications. It is well excited by violet laser at 405 nm, and emits blue/cyan fluorescence light around an emission maximum at ~440 nm, and provides an excellent tool for flow cytometers equipped with a 405 nm violet laser source. This DNA-binding dye might be used for multicolor analysis of live/dead cells with the filter sets of Pacific Blue and BD Horizon V450.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 17549 | 0.5 ml | Price |
Physical properties
| Molecular weight | 744.77 |
| Solvent | DMSO |
Spectral properties
| Excitation (nm) | 371 |
| Emission (nm) | 454 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Instrument settings
| Fluorescence microscope | |
| Excitation | DAPI filter set |
| Emission | DAPI filter set |
| Recommended plate | Black wall/clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 24, 2026

