Phalloidin-iFluor® 647 Conjugate
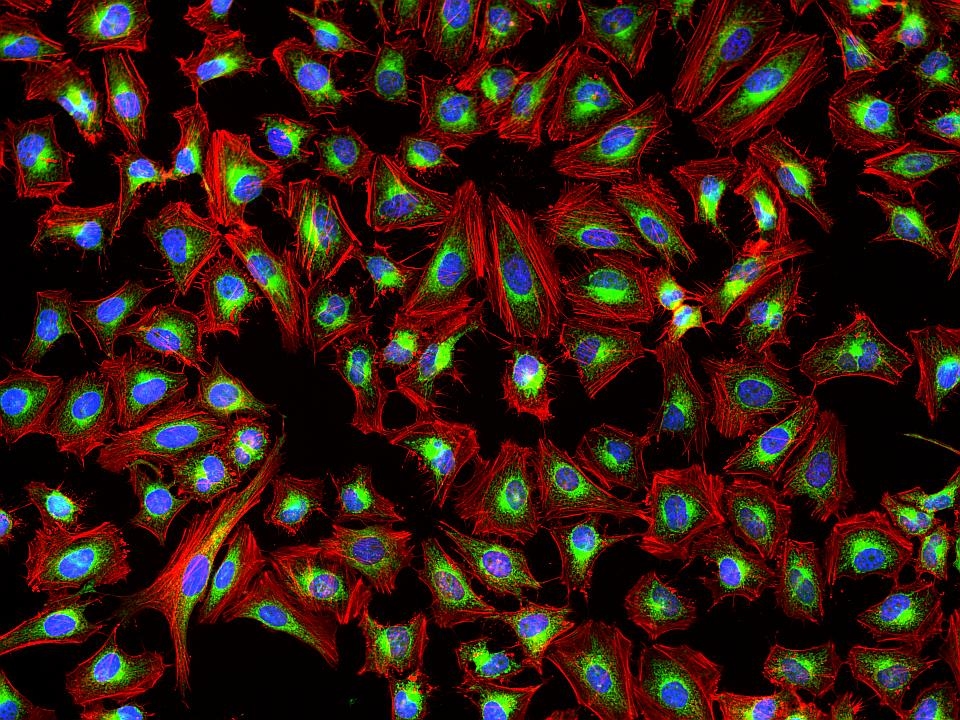
This deep red fluorescent phalloidin conjugate (equivalent to Alexa Fluor® 647-labeled phalloidin) selectively binds to F-actins. Used at nanomolar concentrations, phalloidin derivatives are convenient probes for labeling, identifying and quantitating F-actins in formaldehyde-fixed and permeabilized tissue sections, cell cultures or cell-free experiments. Phalloidin binds to actin filaments much more tightly than to actin monomers, leading to a decrease in the rate constant for the dissociation of actin subunits from filament ends, essentially stabilizing actin filaments through the prevention of filament depolymerization. Moreover, phalloidin is found to inhibit the ATP hydrolysis activity of F-actin. Phalloidin functions differently at various concentrations in cells. When introduced into the cytoplasm at low concentrations, phalloidin recruits the less polymerized forms of cytoplasmic actin as well as filamin into stable "islands" of aggregated actin polymers, yet it does not interfere with stress fibers, i.e. thick bundles of microfilaments. The property of phalloidin is a useful tool for investigating the distribution of F-actin in cells by labeling phalloidin with fluorescent analogs and using them to stain actin filaments for light microscopy. Fluorescent derivatives of phalloidin have turned out to be enormously useful in localizing actin filaments in living or fixed cells as well as for visualizing individual actin filaments in vitro. Fluorescent phalloidin derivatives have been used as an important tool in the study of actin networks at high resolution. AAT Bioquest offers a variety of fluorescent phalloidin derivatives with different colors for multicolor imaging applications.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 23127 | 300 Tests | Price |
Physical properties
| Molecular weight | 1408.65 |
| Solvent | DMSO |
Spectral properties
| Correction factor (260 nm) | 0.03 |
| Correction factor (280 nm) | 0.03 |
| Correction factor (656 nm) | 0.0793 |
| Extinction coefficient (cm -1 M -1) | 250000 1 |
| Excitation (nm) | 656 |
| Emission (nm) | 670 |
| Quantum yield | 0.25 1 |
Storage, safety and handling
| H-phrase | H301, H311, H331 |
| Hazard symbol | T |
| Intended use | Research Use Only (RUO) |
| R-phrase | R23, R24, R25 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on October 9, 2024

