PhosphoWorks™ Fluorimetric Phosphate Assay Kit
Red Fluorescence
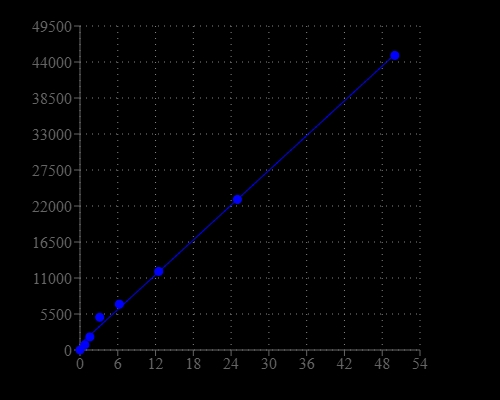
Cells utilize a wide variety of phosphate (Pi) and polyphosphate esters as enzyme substrates, second messengers, membrane structural components and vital energy reservoirs. Phosphate is involved in many biological processes. For example, phosphatases, ATPases and several other enzymes catalyze biochemical reactions in which inorganic phosphate is released from a phosphoester substrate. Detection of many phosphoester-metabolizing enzymes is difficult because suitable substrates are not available. It usually has been necessary to determine inorganic phosphate release using tedious colorimetric assays or radioisotope-based methods. This PhosphoWorks™ Fluorimetric Phosphate Assay Kit has been developed for measuring the activity of any Pi-generating enzyme using our red fluorescent phosphate sensor. The kit provides sensitive detection of Pi, an alternative to hazardous radioactive methods and other less sensitive colorimetric assays. The measurement of Pi is based on the change in the absorbance and fluorescence of our new phosphate sensor. Our kit provides all the essential reagents including phosphate sensor, phosphate standards and assay buffer. The assay is shown to quantitate phosphate in solution at concentrations at least down to 0.1 µM. It can be used to measure the kinetics of phosphate release from phosphatases (such as GTPases and ATPases) by coupling the two enzymatic reactions. The assay can be performed in a convenient 96-well or 384-well microtiter-plate format and easily adapted to automation with no separation steps required.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 21660 | 125 Tests | Price | |
| 21661 | 1250 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 540 nm |
| Emission | 590 nm |
| Cutoff | 570 nm |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 20, 2026
