ReadiPrep™ Lipopolysaccharide (LPS) Isolation Kit
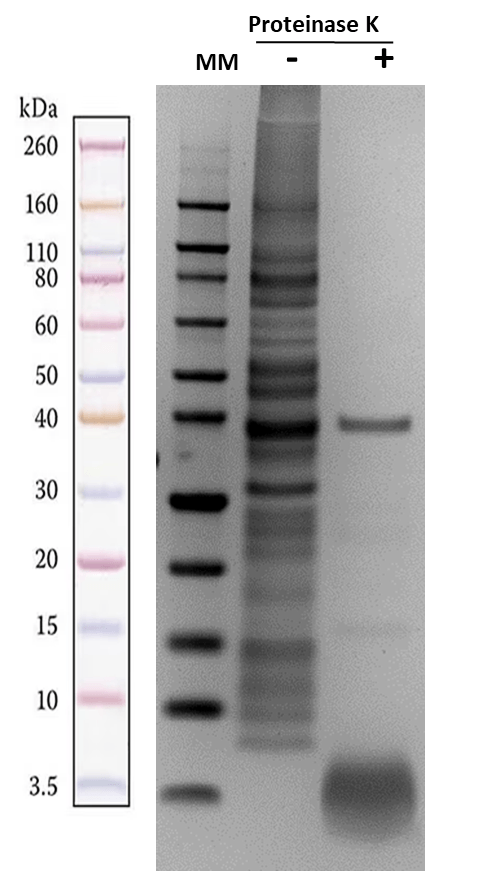
The ReadiPrep™ Lipopolysaccharide (LPS) Isolation Kit provides a safe and effective way to isolate LPS from the outer membrane of Gram-negative bacteria. It utilizes a bacterial membrane lysis buffer and enzymatic protein digestion to extract pure LPS from bacterial cultures, which can be quantified using carbohydrate detection methods. Unlike conventional approaches that rely on hazardous organic chemicals like chloroform or phenol, this kit provides a safer and more time-efficient alternative for isolating high-purity LPS for research applications. Lipopolysaccharides, or LPS, are found in the outer membrane of Gram-negative bacteria. It is a carbohydrate with a low molecular weight of 10-20 kDa. It is a complex molecule made of heterogeneous components comprising O antigen, core oligosaccharide, Lipid A, and non-carbohydrate components such as phosphate and amino acids groups. Lipid A, characterized by its multiple fatty acid chains, anchors the LPS into the bacterial membrane and contributes to the toxicity of the Gram-negative bacteria. Known as endotoxins, LPS is notorious for inducing potent inflammatory responses and sepsis when consumed by animals.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 60201 | 10 Preps | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 18, 2026
