ReadiPrep™ Plasma Membrane Protein Extraction Kit
ReadiPrep™ Plasma Membrane Protein Extraction Kit enables selective isolation of plasma membrane proteins from cultured cells.
- Selective isolation: Enriches plasma membrane proteins with minimal contamination from cytosolic and nuclear components
- Applications: Suitable for Western blotting, ELISA, proteomics, and functional assays
- Streamlined protocol: Eliminates ultracentrifugation and specialized equipment requirements
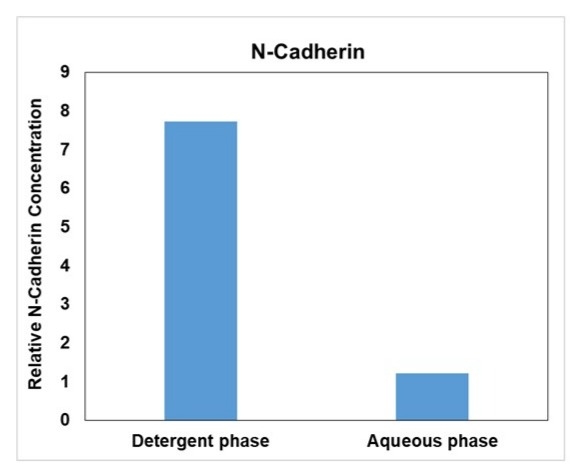
- Comparable alternative: Equivalent to Thermo Fisher’s Mem-PER™ Plus Membrane Protein Extraction Kit

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 60209 | 50 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 11, 2026
