ReadiPrep™ Protein A-Agarose Resin
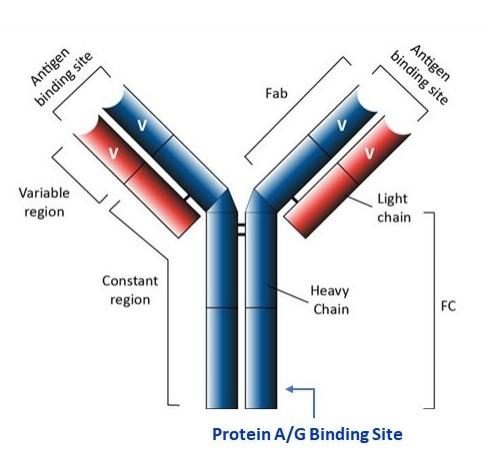
ReadiPrep™ Protein-A Agarose Resin was prepared by covalently immobilizing protein A onto high-quality crosslinked beaded agarose. These agarose beads possess physical and chemical characteristics that make them suitable for use in various batch or column-type affinity purification procedures. Our ReadiPrep™ Protein-A Agarose Resin has four high-affinity binding sites that interact with the Fc region of IgG-class antibodies from selected mammalian species. Protein A agarose has pH stability from 3 to 13.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 55000 | 1 mL | Price | |
| 55005 | 5 mL | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Refrigerated (2-8 °C) |
| UNSPSC | 12352200 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on March 3, 2026
