Screen Quest™ Colorimetric Chloride Channel Assay Kit
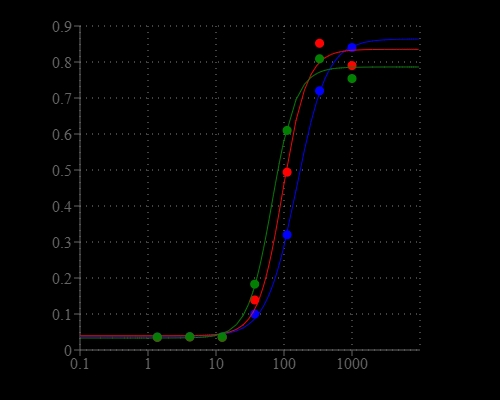
Chloride channels have a variety of important physiological and cellular functions that include regulation of pH, volume homeostasis, organic solute transport, cell migration, cell proliferation and differentiation. Chloride channels represent valuable drug targets. A number of chronic disease states such as cystic fibrosis and Bartter's syndrome are due to defects in chloride channel functions. However, the existing technologies for screening chloride channel modulators are a compromise between throughput, sensitivity and physiological relevance. Screen Quest™ Colorimetric Chloride Channel Assay Kit provides a sensitive and robust colorimetric method for studying chloride channels. The assay is based on our proprietary iodide indicator (Iodide Blue™) for measuring iodide concentration, as low as 30 nM of iodide was detected by this assay. Screen Quest™ Chloride Channel Assay Kit provides an optimized assay method for monitoring chloride channels. The assay can be performed in a convenient 96-well or 384-well microtiter-plate format and easily adapted to automation.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 36350 | 10 Plates | Price | |
| 36351 | 100 Plates | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Absorbance microplate reader | |
| Absorbance | 630, 380, or 405 nm |
| Recommended plate | Clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on March 8, 2026
