Screen Quest™ Colorimetric Glucose Uptake Assay Kit
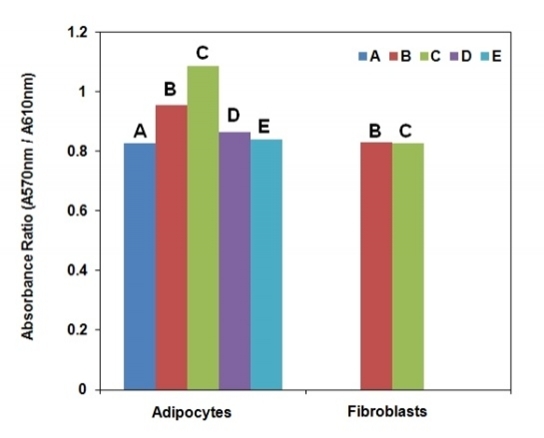
Glucose transport systems are responsible for transporting glucose across cell membranes. Measuring uptake of 2-deoxyglucose (2-DG), a glucose analog, in tissues and cells is widely accepted as a reliable method to estimate the amount of glucose uptake and to investigate the regulation of glucose metabolism and mechanism of insulin resistance. The 2-DG uptake is commonly determined by using non-metabolized 2-DG labeled with tritium or C14. However, routine use of a radiolabelled probe is costly and requires a tedious special handling procedure. AAT Bioquest's Screen Quest™ Colorimetric Glucose Uptake Assay Kit provides a sensitive and non-radioactive assay in tissues or cultured cells. In this assay 2-DG is taken up by glucose transporters, and metabolized to 2-DG-6-phosphate (2-DG6P). The non-metabolizable 2-DG6P accumulates in the cells, and is proportional to glucose uptake by cells. The accumulated 2-DG6P is enzymatically oxidized and generates NADPH, which is specifically monitored by a chromogenic NADPH sensor. The signal can be read by a absorption microplate reader by reading the OD ratio at wavelength 570 nm to 610 nm.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 36503 | 100 Tests | Price | |
| 36504 | 500 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Absorbance microplate reader | |
| Absorbance | 570/610 nm |
| Recommended plate | Clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 27, 2026
