Screen Quest™ Rhod-4 No Wash Calcium Assay Kit
Medium Removal
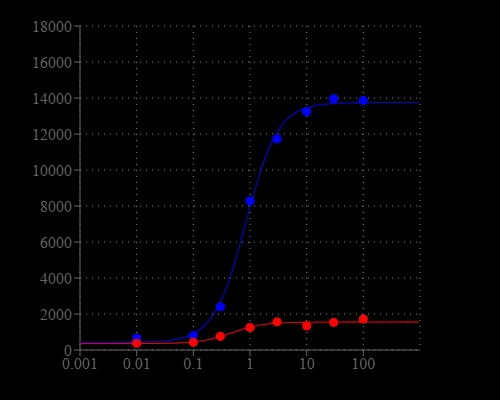
Calcium flux assays are preferred methods in drug discovery for screening G protein coupled receptors (GPCR). Screen Quest™ Rhod-4 NW Calcium Assay Kit provides a homogeneous fluorescence-based assay for detecting the intracellular calcium mobilization. Cells expressing a GPCR of interest that signals through calcium are pre-loaded with our proprietary Rhod-4 NW which can cross cell membrane. Rhod-4 is the brightest red calcium indicator available for HTS screening. Once inside the cell, the lipophilic blocking groups of Rhod-4™ are cleaved by non-specific cell esterase, resulting in a negatively charged fluorescent dye that stays inside cells, and its fluorescence is greatly enhanced upon binding to calcium. When cells stimulated with screening compounds, the receptor signals release of intracellular calcium, which greatly increase the fluorescence of Rhod-4. The characteristics of its long wavelength, high sensitivity, and >250 times fluorescence increases (when it forms complexes with calcium) make Rhod-4™ an ideal indicator for measurement of cellular calcium. This Screen Quest Rhod-4 NW Calcium Assay Kit provides an optimized assay method for monitoring G-protein-coupled receptors (GPCRs) and calcium channels. The assay can be performed in a convenient 96-well or 384-well microtiter-plate format and easily adapted to automation.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 36330 | 1 Plate | Price | |
| 36331 | 10 Plates | Price | |
| 36332 | 100 Plates | Price |
Spectral properties
| Excitation (nm) | 523 |
| Emission (nm) | 551 |
| Quantum yield | 0.1 1 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 540 nm |
| Emission | 590 nm |
| Cutoff | 570 nm |
| Recommended plate | Black wall/clear bottom |
| Instrument specification(s) | Bottom read mode/Programmable liquid handling |
| Other instruments | ArrayScan, FDSS, FLIPR, FlexStation, IN Cell Analyzer, NOVOStar, ViewLux |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 19, 2026

