Signal Guard™ HRP reaction stopping reagent
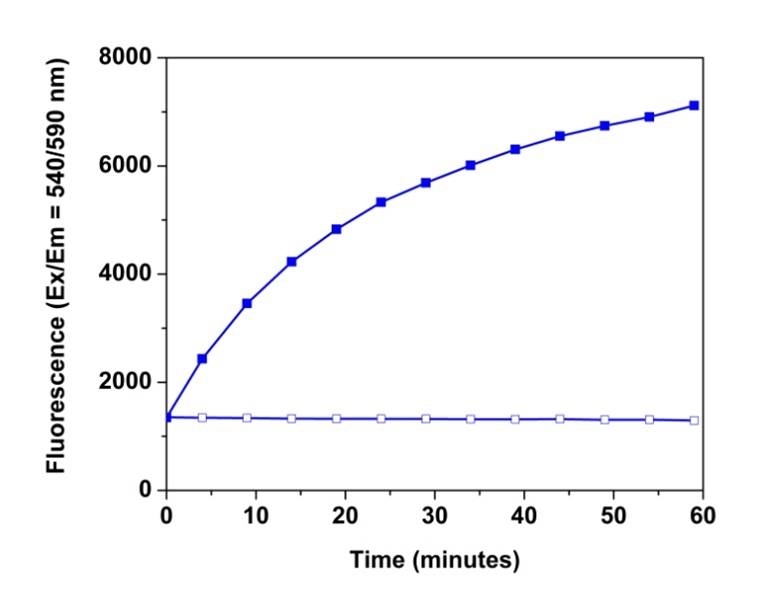
HRP coupling reactions provide sensitive biomolecular assays based on hydrogen peroxide- generating enzyme systems linked to peroxidase- mediated oxidation. Fluorogenic HRP substrates are preferred to use for enhancing assay sensitivities. Among them, the most commonly used HRP substrates include ADHP (also called Amplex® Red, #11000), Amplex® UltraRed and Amplite® Red. Typically, detection reactions are performed in microplate wells and are initiated by adding a fluorogenic HRP substrate, resulting in a continuous fluorescence increase. It is critical to ensure that the timing of the standard and unknown sample measurements is the same. Our Signal Guard™ HRP reaction stopping reagent provides convenience and control by allowing the fluorescence signal-generating reaction to be terminated at a user-determined time point. After addition of the stop reagent, the fluorescence signal remains stable. The Signal Guard™ HRP reaction stopping reagent is designed for use in conjunction with ADHP (Amplex® Red ), Amplite® and Amplex®UltraRed fluorogenic substrates. Under the same conditions, Signal Guard™ HRP reaction stopping reagent significantly outperforms the Amplex® Red/UltraRed Stop Reagent (#A33855) from ThermoFisher. Our Signal Guard™ HRP reaction stopping reagent can also be used in other HRP reaction systems.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 11020 | 500 Tests | Price |
Physical properties
| Solvent | Water |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 21, 2026
