Tide Fluor™ 5WS acid
TF5WS acid; Superior replacement for Cy5
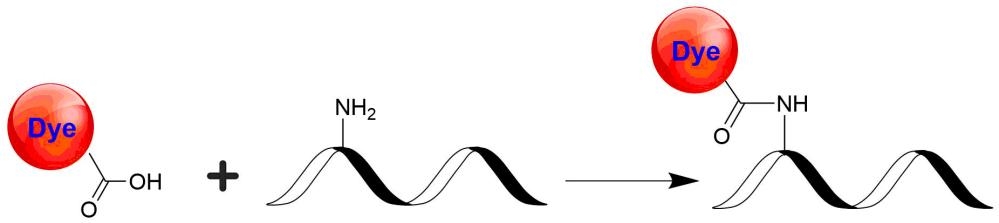
Tide Fluor™ 5WS (TF5WS) family has the spectral properties essentially identical to those of Cy5. Compared to Cy5 probes TF5WS family has much stronger fluorescence and higher photostability. Additionally their fluorescence is pH-independent from pH 3 to 11. These characteristics make this new dye family a superior alternative to Cy5. TF5WS-labeled peptides and nucleotides exhibit much stronger fluorescence and higher photostability than the ones labeled with Cy5. In pairing with our Tide Quencher™ 5WS (TQ5WS), a variety of FRET peptides and nucleotides can be developed for detecting proteases and molecular beacons with enhanced sensitivity and stability.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 2278 | 10 mg | Price |
Physical properties
| Molecular weight | 864.92 |
| Solvent | DMSO |
Spectral properties
| Correction factor (280 nm) | 0.027 |
| Extinction coefficient (cm -1 M -1) | 250000 |
| Excitation (nm) | 649 |
| Emission (nm) | 663 |
| Quantum yield | 0.27 1 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 20, 2026

