Tide Quencher™ 2WS alkyne
TQ2WS alkyne
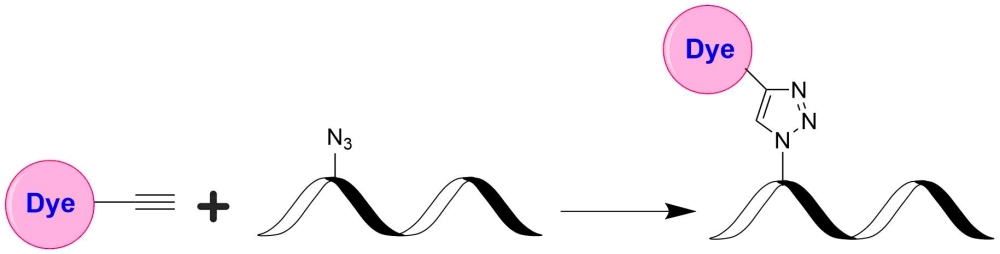
TQ2WS is designed to be a superior quencher to FAM, HEX, TET, JOE, TF2 and rhodamine 6G. TQ2WS has (a). much stronger absorption; (b). much higher quenching efficiency; and (c). versatile reactive forms with desired solubility for labeling oligonucleotides and peptides. This TQ2WS product is reactive to azides, and useful for click chemistry. TQ2WS has much higher water solubility than TQ2. We recommend that you use TQ2WS for the applications that require good water solubility.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 2213 | 1 mg | Price | |
| 2214 | 5 mg | Price |
Physical properties
| Molecular weight | 527.66 |
| Solvent | DMSO |
Spectral properties
| Absorbance (nm) | 541 |
| Correction factor (260 nm) | 0.100 |
| Correction factor (280 nm) | 0.120 |
| Extinction coefficient (cm -1 M -1) | 48000 |
Storage, safety and handling
| Intended use | Research Use Only (RUO) |
| Storage | Freeze (< -15 °C); Minimize light exposure |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 21, 2026

