Transfectamine™ mRNA Transfection Reagent
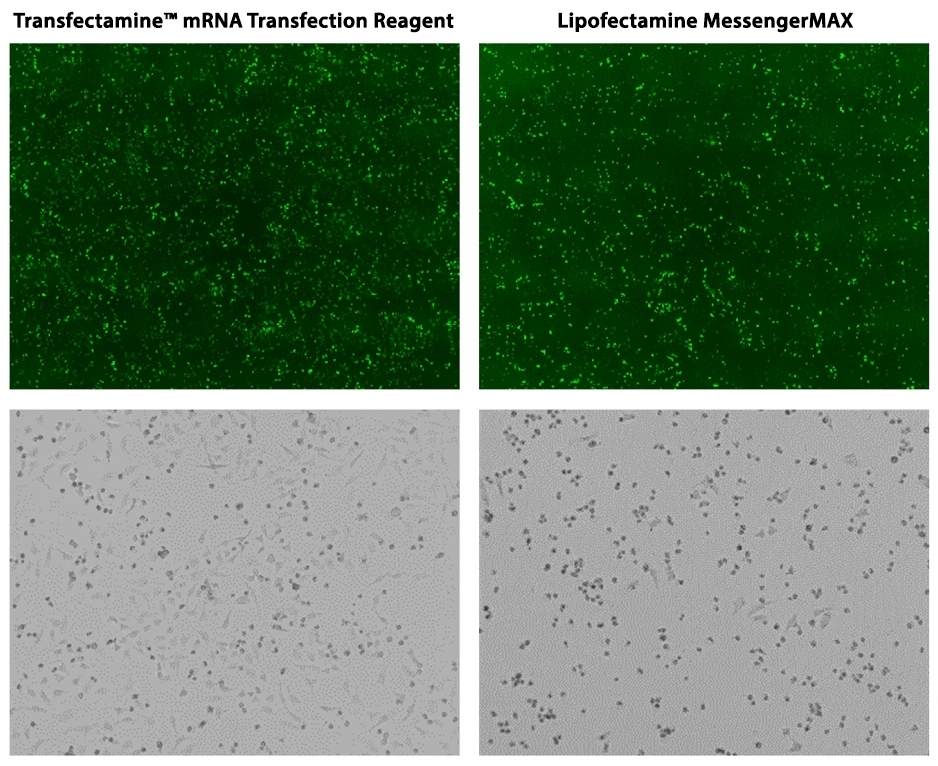
Transfectamine™ mRNA Transfection Reagent is a powerful and versatile transfection reagent designed to introduce a higher amount of mRNA into eukaryotic cells, or more specifically, into animal cells. It delivers high transfection efficiency in a wide variety of adherent and suspension cell lines, including difficult-to-transfect cells. Nuclear uptake is not required, which results in faster protein expression than DNA transfection without the risk of genomic integration. The low toxicity of Transfectamine™ mRNA Transfection Reagent allows higher viability of transfected cells. Transfectamine™ mRNA Transfection Reagent does not require special medium and is easier to use compared to most of the commercial transfection reagents.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 60029 | 50 uL | Price | |
| 60030 | 0.5 mL | Price | |
| 60031 | 5 mL | Price |
Physical properties
| Solvent | Water |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 30, 2026
