trFluor™ Tb maleimide
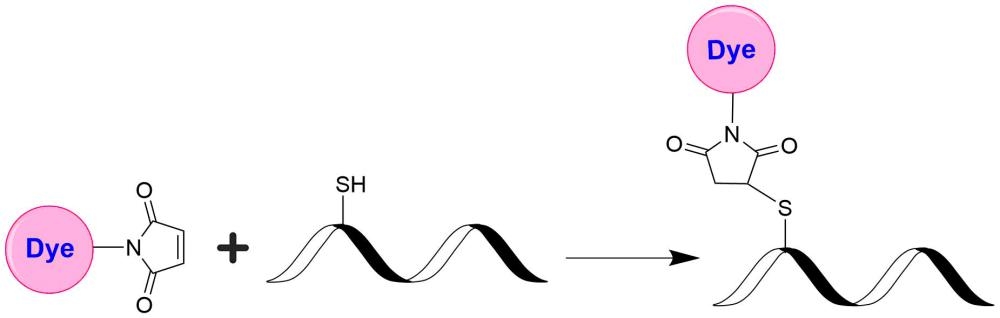
Many biological compounds present in cells, serum or other biological fluids are naturally fluorescent, and thus the use of conventional, prompt fluorophores leads to serious limitations in assay sensitivity due to the high background caused by the autofluorescence of the biological molecules to be assayed. The use of long-lived fluorophores combined with time-resolved detection (a delay between excitation and emission detection) minimizes prompt fluorescence interferences. Our trFluor™ Tb probes enable time-resolved fluorometry (TRF) for the assays that require high sensitivity. These trFluor™ Tb probes have large Stokes shifts and extremely long emission half-lives when compared to more traditional fluorophores such as Alexa Fluor or cyanine dyes. Compared to the other TRF compounds, our trFluor™ Tb probes have relatively high stability, high emission yield and ability to be linked to biomolecules. Moreover, our trFluor™ Tb probes are insensitive to fluorescence quenching when conjugated to biological polymers such as antibodies.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 1444 | 100 ug | Price |
Physical properties
| Molecular weight | 1577.75 |
| Solvent | DMSO |
Spectral properties
| Correction factor (260 nm) | 0.942 |
| Correction factor (280 nm) | 0.797 |
| Excitation (nm) | 333 |
| Emission (nm) | 544 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on March 3, 2026

