Amplite® Fluorimetric Glutathione Peroxidase Assay Kit *Blue Fluorescence*
| Price | |
| Catalog Number | |
| Unit Size | |
| Quantity |
| Telephone | 1-800-990-8053 |
| Fax | 1-800-609-2943 |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Shipping | Standard overnight for United States, inquire for international |
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
| Overview |
Platform
Fluorescence microplate reader
| Excitation | 420 nm |
| Emission | 480 nm |
| Cutoff | 430 nm |
| Recommended plate | Solid black |
Components
Example protocol
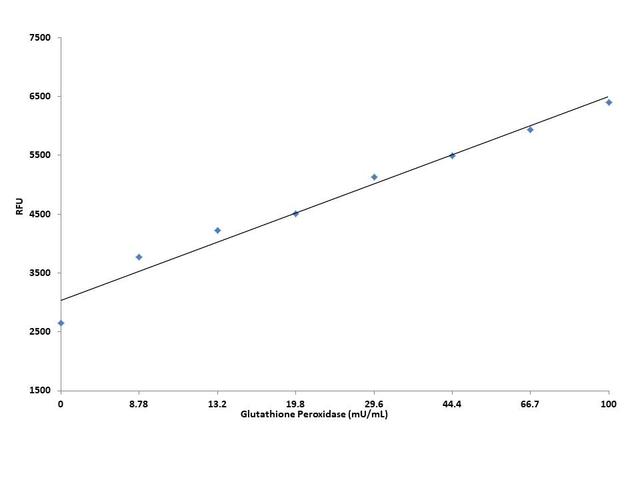
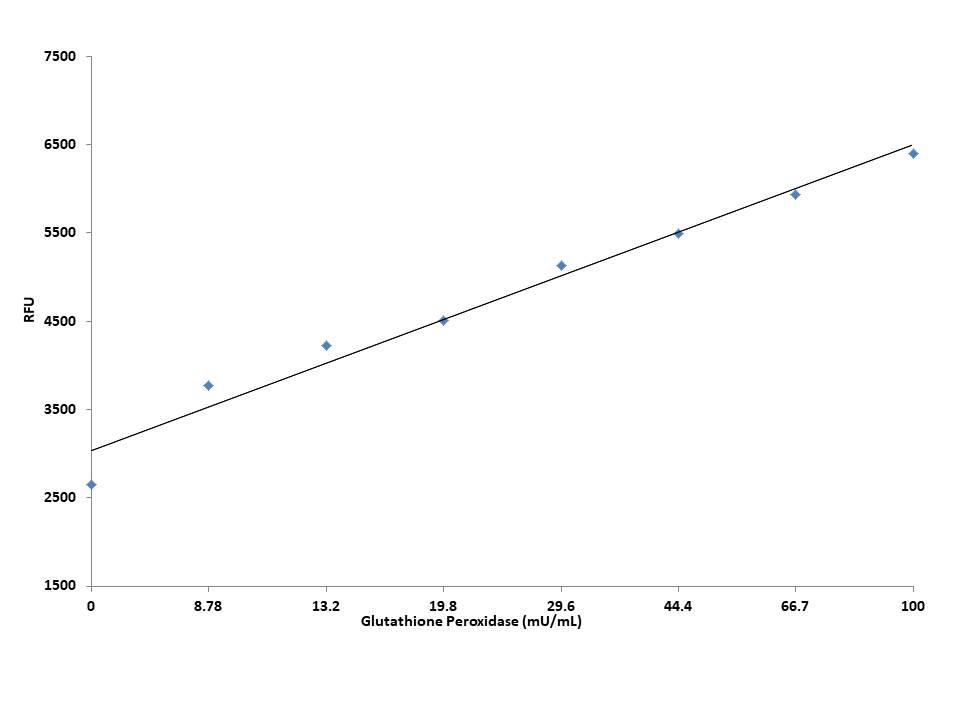
AT A GLANCE
- Prepare GPx standards or test samples (50 µL)
- Add GPx working solution (50 µL)
- Incubate at room temperature for 30 min
- Add 20 µL Quest Fluor™ NADP Probe
- Add 20 µL NADP Assay Solution
- Incubate at room temperature for 10 - 20 min
- Add 15 µL Enhancer Solution
- Incubate at room temperature for 30 - 60 min
- Record Fluorescence at Ex/Em= 420/480nm (Cutoff = 430 nm)
To achieve the best results, it’s strongly recommended to use the black plates. Thaw one vial of each kit component at room temperature before starting the experiment.
PREPARATION OF STOCK SOLUTIONS
Unless otherwise noted, all unused stock solutions should be divided into single-use aliquots and stored at -20 °C after preparation. Avoid repeated freeze-thaw cycles
Add 50 µL of ddH2O or 1× PBS buffer into the vial of GPx standard (Component A) to make 10 U/mL standard solution.
Add 100 µL of ddH2O into the vial of GSH (Component D) to make 100X GSH stock solution.
Add 100 µL of ddH2O into the vial of substrate (Component E) to make 100X GPx Substrate stock solution.
PREPARATION OF STANDARD SOLUTIONS
https://www.aatbio.com/tools/serial-dilution/11560
PREPARATION OF WORKING SOLUTION
- Add 5 mL of Assay Buffer (Component B) into a bottle of Enzyme Mix (Component C).
- Add 50 µL GSH stock solution (Component D), 50 µL GPx Substrate stock solution (Component E) into the bottle of Component B+C, and mix well to make GPx working solution (Component B+C+D+E). Note: This GPx working solution is enough for one 96-well plate. It is not stable; please use it promptly. It is not recommend storing unused GPx working solution.
SAMPLE EXPERIMENTAL PROTOCOL
Table 1. Layout of GPx standards and test samples in a solid black 96-well microplate. GP= GPx Standards (GP1 - GP7, 8.78 to 100 mU/mL), BL=Blank Control, TS=Test Samples.
| BL | BL | TS | TS |
| GP1 | GP1 | ... | ... |
| GP2 | GP2 | ... | ... |
| GP3 | GP3 | ||
| GP4 | GP4 | ||
| GP5 | GP5 | ||
| GP6 | GP6 | ||
| GP7 | GP7 |
Table 2. Reagent composition for each well.
| Well | Volume | Reagent |
| GP1 - GP7 | 50 µL | Serial Dilution (8.78 to 100 mU/mL) |
| BL | 50 µL | 1X PBS Buffer + 0.1% BSA |
| TS | 50 µL | Test Sample |
- Prepare GPx standards (GP), blank controls (BL), and test samples (TS) according to the layout provided in Tables 1 and 2. For a 384- well plate, use 25 µL of reagent per well instead of 50 µL.
- Add 50 µL of GPx working solution to each well of GPx standard, blank control, and test samples to make the total assay volume of 100 µL/well. For a 384-well plate, add 25 µL GPx working solution into each well instead, for total of 50 µL/well.
- Incubate the reaction at room temperature for 30 minutes, protected from light.
- Add 20 µL Quest Fluor™ NADP Probe (Component F) into each well of GPx standard, blank control, and test samples, mix well.
- Add 20 µL NADP Assay Solution (Component G) into each well, mix well. For a 384-well plate, add 25 µL of sample and 10 µL of Quest Fluor™ NADP Probe (Component F) and 10 µL NADP Assay Solution (Component G) into each well.
- Incubate the reaction at room temperature for 10 - 20 minutes, protected from light.
- Add 15 µL Enhancer (Component H) to each well to make the total assay volume of 155 µL/well, and incubate at room temperature for 30 - 60 minutes, protected from light. For a 384-well plate, add 7.5 µL Enhancer.
- Monitor the fluorescence increase with a fluorescence plate reader at Ex/Em = 420/480 nm (Cutoff = 430nm).
Product Family
| Name | Excitation (nm) | Emission (nm) |
| Amplite® Fluorimetric Peroxidase (HRP) Assay Kit *Red Fluorescence* | 571 | 584 |
| Amplite® Fluorimetric Peroxidase (HRP) Assay Kit *Near Infrared Fluorescence* | 648 | 668 |
Images
Citations
Authors: Zhang, Kai and Nakamura, Satoshi and Furukawa, Seiichi
Journal: Insects (2023): 602
Authors: Wake, Hidenori and Takahashi, Yohei and Yoshii, Yukinori and Gao, Shangze and Mori, Shuji and Wang, Dengli and Teshigawara, Kiyoshi and Nishibori, Masahiro
Journal: Free Radical Research (2020): 649--661
References
Authors: Wang QY, Liu ZS, Wang J, Wang HX, Li A, Yang Y, Wang XZ, Zhao YQ, Han QY, Cai H, Liang B, Song N, Li WH, Li T.
Journal: Biochem Biophys Res Commun (2014): 454
Authors: Yu Y, Song J, Guo X, Wang S, Yang X, Chen L, Wei J.
Journal: Free Radic Biol Med (2014): 332
Authors: Miao L, Zhang X, Si C, Gao Y, Zhao L, Hou C, Shoseyov O, Luo Q, Liu J.
Journal: Org Biomol Chem (2014): 362
Authors: Ianiski FR, Alves CB, Bassaco MM, Silveira CC, Luchese C.
Journal: J Pharm Pharmacol. (2014)
Authors: Koretsi V, Kirschneck C, Proff P, Romer P.
Journal: Eur J Orthod. (2014)
Authors: Sakamoto T, Maebayashi K, Nakagawa Y, Imai H.
Journal: Genes Cells (2014): 778
Authors: Barbosa KB, Volp AC, Marques-Rocha JL, Ribeiro SM, Navarro-Blasco I, Zulet MA, Martinez JA, Bressan J.
Journal: Redox Rep (2014): 251
Authors: Yang S, Jensen MK, Rimm EB, Willett W, Wu T.
Journal: Am J Epidemiol (2014): 901
Authors: Guo X, Yu Y, Liu X, Zhang Y, Guan T, Xie G, Wei J.
Journal: IUBMB Life. (2014)
Authors: Barroso M, Florindo C, Kalwa H, Silva Z, Turanov AA, Carlson BA, de Almeida IT, Blom HJ, Gladyshev VN, Hatfield DL, Michel T, Castro R, Loscalzo J, H and y DE., undefined
Journal: J Biol Chem (2014): 15350
Application notes
Acetylcholinesterase Inhibitory Activity of Pigment Echinochrome A
Ameliorative Effect of Novel Vitamin Formula with Herbal Extracts on Scopolamine-Induced Alzheimer's Disease
An Increase in Plasma Homovanillic Acid with Cocoa Extract Consumption Is Associated with the Alleviation of Depressive Symptoms in Overweight or Obese Adults
Attenuation of lysyl oxidase and collagen gene expression in keratoconus patient corneal epithelium corresponds to disease severity