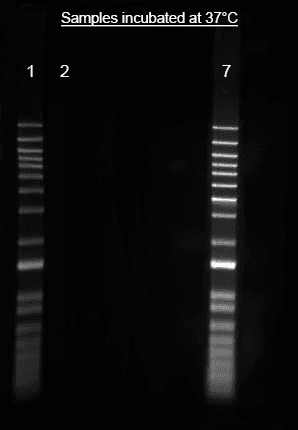
Helixyte™ iFluor® 488 Nucleic Acid Labeling Dye *Optimized for Labeling 2x100 ug DNA/RNA*
| Price | |
| Catalog Number | |
| Unit Size | |
| Quantity |
| Telephone | 1-800-990-8053 |
| Fax | 1-800-609-2943 |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Shipping | Standard overnight for United States, inquire for international |
| Solvent | DMSO |
| Correction Factor (260 nm) | 0.21 |
| Correction Factor (280 nm) | 0.11 |
| Extinction coefficient (cm -1 M -1) | 750001 |
| Excitation (nm) | 491 |
| Emission (nm) | 516 |
| Quantum yield | 0.91 |
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| Overview |
Correction Factor (260 nm) 0.21 | Correction Factor (280 nm) 0.11 | Extinction coefficient (cm -1 M -1) 750001 | Excitation (nm) 491 | Emission (nm) 516 | Quantum yield 0.91 |
Example protocol
AT A GLANCE
Combine DNA with the Helixyte™ iFluor® 488 Nucleic Acid Labeling Dye stock solution.
Incubate for 1 hour at 37°C.
Purify the conjugate as required for downstream applications.
PREPARATION OF STOCK SOLUTIONS
Unless otherwise noted, all unused stock solutions should be divided into single-use aliquots and stored at -20 °C after preparation. Avoid repeated freeze-thaw cycles
Before opening the vial, thaw Helixyte™ iFluor® nucleic acid labeling dye at room temperature. Briefly centrifuge to collect the dried pellet.
Add 70 μL of DMSO to the Helixyte™ iFluor® 488 Nucleic Acid Labeling Dye vial to prepare a 10 mM stock solution.
Note: It is recommended to divide any unused stock solution into single-use aliquots. Store the aliquots at ≤-20 ºC and protect them from light. Avoid repeated freeze-thaw cycles.
SAMPLE EXPERIMENTAL PROTOCOL
Prepare the labeling reaction according to the specifications in table 1 below.
Table 1. Standard Nucleic Acid Labeling Reaction.
Components Volume added to reaction Final Concentration DNA (1 mg/mL) 2 to 5 µL 2 to 5 µg Helixyte™ iFluor® 488 Nucleic acid Labeling Dye stock solution 1 µL 100 µM TE Buffer (pH 8 to 8.5) Add sufficient buffer to adjust the volume to 100 µL Note: This DNA:Dye ratio results in labeling efficiencies that are appropriate for most applications. The amount of Helixyte™ iFluor® 488 Nucleic Acid Labeling Dye or the reaction incubation time can be adjusted to modify the labeling density as per the application requirements. The DNA-to-dye ratio must be optimized to achieve a higher labeling ratio.
Incubate the reaction at 37℃ for 1 hour, protected from light.
Note: After 30 minutes of incubation, briefly centrifuge the reaction to minimize the effects of evaporation and maintain the appropriate concentration of the reaction components.
Note: Alternatively, the reaction can be incubated at room temperature for 2 hours. For the best labeling condition, we recommend incubating at 37℃.
After incubation, the labeling mix can be purified to remove any free labeling dye. Refer to the “Purification of labeling mix with alcohol precipitation” section below for instructions.
Add 0.1 volume (10 uL) of 5M sodium chloride and 2 - 2.5 volumes of ice-cold 100% ethanol (250 uL) to the reaction. Mix well and place at ≤ -20°C for at least 30 minutes.
Centrifuge at full speed (>14,000 x g) in a refrigerated micro centrifuge for 15-30 minutes to pellet the labeled nucleic acid. Once pelleted, carefully remove the ethanol with a micropipette. Do not disturb the pellet.
Note: Small nucleic acid quantities can be difficult to visualize. Mark and orient the precipitate-containing tubes in the microfuge such that the pellet will form in a predetermined place.
Wash the pellet once with 500 μL of room temperature 70% ethanol. Centrifuge at full speed for an additional 15-30 minutes.
Remove all traces of ethanol with a micropipette. DO NOT allow the sample to dry longer than 5 minutes as the pellet may become difficult to resuspend.
Resuspend the labeled DNA with ~ 30 µL sterile water.
Store the purified, labeled nucleic acid for long-term storage or put on ice for immediate use.
Spectrum
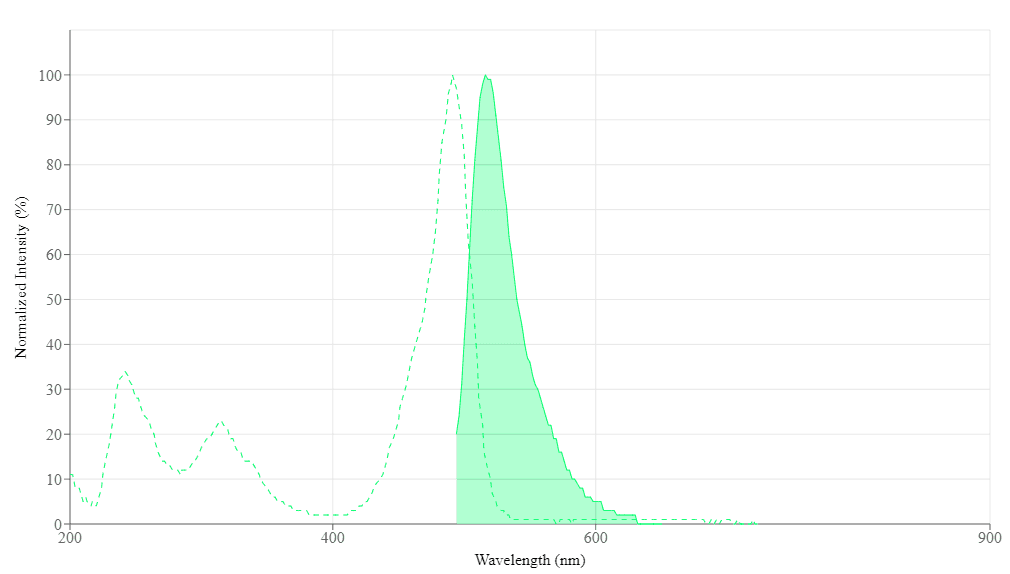
Spectral properties
| Correction Factor (260 nm) | 0.21 |
| Correction Factor (280 nm) | 0.11 |
| Extinction coefficient (cm -1 M -1) | 750001 |
| Excitation (nm) | 491 |
| Emission (nm) | 516 |
| Quantum yield | 0.91 |
Product Family
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Quantum yield | Correction Factor (260 nm) | Correction Factor (280 nm) |
| Helixyte™ iFluor® 350 Nucleic Acid Labeling Dye *Optimized for Labeling 2x100 ug DNA/RNA* | 345 | 450 | 200001 | 0.951 | 0.83 | 0.23 |
| Helixyte™ iFluor® 555 Nucleic Acid Labeling Dye *Optimized for Labeling 2x100 ug DNA/RNA* | 557 | 570 | 1000001 | 0.641 | 0.23 | 0.14 |
| Helixyte™ iFluor® 594 Nucleic Acid Labeling Dye *Optimized for Labeling 2x100 ug DNA/RNA* | 587 | 603 | 2000001 | 0.531 | 0.05 | 0.04 |
| Helixyte™ iFluor® 647 Nucleic Acid Labeling Dye *Optimized for Labeling 2x100 ug DNA/RNA* | 656 | 670 | 2500001 | 0.251 | 0.03 | 0.03 |
| Helixyte™ iFluor® 750 Nucleic Acid Labeling Dye *Optimized for Labeling 2x100 ug DNA/RNA* | 757 | 779 | 2750001 | 0.121 | 0.044 | 0.039 |
Images
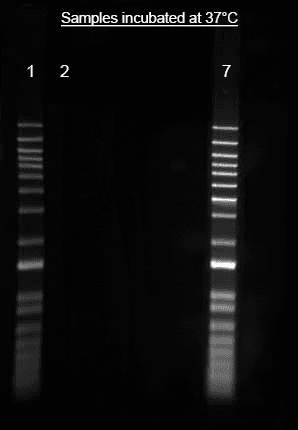
References
Authors: Oka, Yoshimi
Journal: ACS omega (2023): 1109-1113
Authors: Akamatsu, Ken and Satoh, Katsuya and Shikazono, Naoya and Saito, Takeshi
Journal: Radiation research (2023)
Authors: Pavluch, Vojtěch and Špaček, Tomáš and Engstová, Hana and Dlasková, Andrea and Ježek, Petr
Journal: Scientific reports (2023): 5788
Authors: de Sousa Portilho, Adrhyann Jullyanne and da Silva, Emerson Lucena and Bezerra, Emanuel Cintra Austregésilo and Moraes Rego Gomes, Carinne Borges de Souza and Ferreira, Vitor and de Moraes, Maria Elisabete Amaral and da Rocha, David Rodrigues and Burbano, Rommel Mário Rodriguez and Moreira-Nunes, Caroline Aquino and Montenegro, Raquel Carvalho
Journal: International journal of molecular sciences (2022)
Authors: Guha, Souvik and Yussif El-Deeb, Ibrahim and Yadav, Shalini and Das, Ranajit and Dutta Dubey, Kshatresh and Baruah, Mousumi and Gremaud, Ludovic and Sen, Subhabrata
Journal: Chemistry (Weinheim an der Bergstrasse, Germany) (2022): e202202405
Authors: Zhao, Xue Zhi and Kiselev, Evgeny and Lountos, George T and Wang, Wenjie and Tropea, Joseph E and Needle, Danielle and Hilimire, Thomas A and Schneekloth, John S and Waugh, David S and Pommier, Yves and Burke, Terrence R
Journal: Chemical science (2021): 3876-3884
Authors: Akamatsu, Ken and Shikazono, Naoya and Saito, Takeshi
Journal: Analytical and bioanalytical chemistry (2021): 1185-1192
Authors: Huang, Yuyang and Deng, Liyun and Su, Di and Huang, Xiangyi and Ren, Jicun
Journal: The Analyst (2021)
Authors: Zhang, Yan and Hua, Ruo-Nan and Zhang, Chun-Yang
Journal: Analytical chemistry (2020): 4700-4706
Authors: Wang, Cun and Chen, Min and Han, Qian and Wu, Jingling and Zhao, Xin and Fu, Yingzi
Journal: Biosensors & bioelectronics (2020): 112146