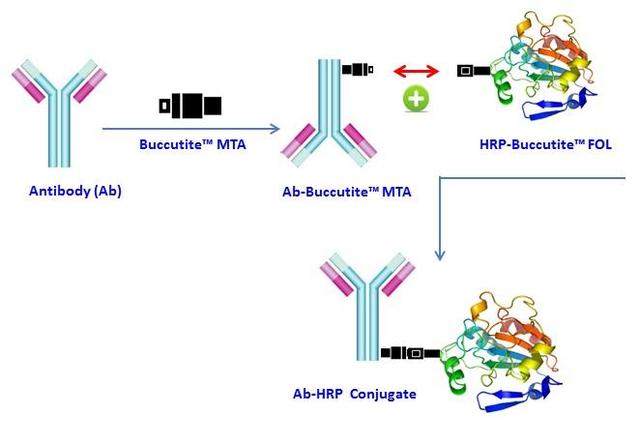
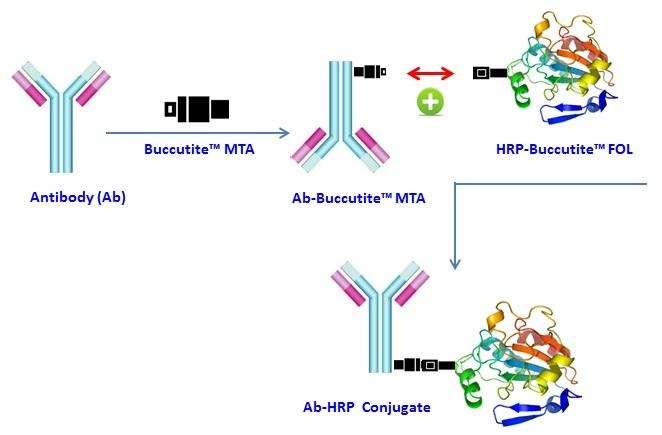
Buccutite™ Peroxidase (HRP) Antibody Conjugation Kit *Optimized for Labeling 1 mg Protein*
Example protocol
AT A GLANCE
Add 10 µL Reaction Buffer (Component C) into antibody solution (200 µL) if antibody concentration is 5 mg/mL in PBS
Add 10 µL DMSO to Buccutite™ MTA vial (Component B) to make Buccutite™ MTA working solution
Add 4.5 µL Buccutite™ MTA stock solution into the antibody solution
- Incubate at room temperature for 30 minutes
Add 500 µL H2O to reconstitute Buccutite™ FOL-Activated HRP (Component A)
Mix the Buccutite™ MTA activated antibody solution with 500 µL Buccutite™ FOL-Activated HRP (Component A)
Incubate at room temperature for 60 minutes
Before opening the vials, all components should be warmed to room temperature and briefly centrifuged. The required solutions should be immediately prepared before starting the conjugation process. The following is an example SOP for labeling goat anti-mouse IgG antibody.
PREPARATION OF STOCK SOLUTIONS
Unless otherwise noted, all unused stock solutions should be divided into single-use aliquots and stored at -20 °C after preparation. Avoid repeated freeze-thaw cycles
Add 10 µL of DMSO into the vial of Buccutite™ MTA (Component B).
Note: This stock solution should be used promptly.
PREPARATION OF WORKING SOLUTION
To label 1 mg of antibody (assuming the target antibody concentration is 5 mg/mL), mix 10 µL of the Reaction Buffer (Component C) with 200 µL of the target antibody solution. If your antibody is not 5 mg/mL, please add 5% of the total volume of the Reaction Buffer (Component C).
Note: The protocol assumes the target antibody concentration is 5 mg/mL. The antibody –Buccutite™ MTA reaction efficiency is significantly reduced if the antibody concentration is less than 1 mg/mL. For optimal labeling efficiency, the antibody concentration range of 1-5 mg/mL is recommended.
Note: The antibody should be dissolved in 1X phosphate-buffered saline (PBS), pH 7.2-7.4. If the antibody is dissolved in glycine buffer, it must be dialyzed against 1X PBS, pH 7.2-7.4, or use ReadiUse™ 10KD Spin Filter (Cat. # 60502 from AAT Bioquest) to remove free amines or ammonium salts (such as ammonium sulfate and ammonium acetate) that are widely used for antibody precipitation.
Note: Impure antibodies or antibodies stabilized with bovine serum albumin (BSA) or gelatin will not be labeled well.
SAMPLE EXPERIMENTAL PROTOCOL
Add 4.5 µL Buccutite ™ MTA stock solution to antibody working solution, and mix them well by repeatedly pipetting for a few times or vortex the vial for a few seconds.
Keep the antibody- Buccutite ™ MTA reaction mixture at room temperature for 30 - 60 minutes.
Note: The antibody-Buccutite™ MTA reaction mixture can be rotated or shaken for a longer time if desired.
Make HRP- Buccutite™ FOL solution by adding 500 µL ddH2O into the vial of Buccutite™ FOL-Activated HRP (Component A), mix well by repeatedly pipetting for a few times or vortex the vial for a few seconds.
Mix whole vial of Buccutite™ FOL-Activated HRP solution into the antibody- Buccutite™ MTA solution, mix well and rotating the mixture for 1 hour at room temperature.
The HRP-antibody conjugate is now ready to use. Optional: HRP-antibody conjugate can be further purified through size exclusion chromatography to get better performance.
Note: Alternatively, add antibody-Buccutite™ MTA solution mixture to the vial of Buccutite™ FOL-Activated HRP directly.
The antibody conjugate should be stored at > 0.5 mg/mL in the presence of a carrier protein (e.g., 0.1% bovine serum albumin). The HRP-Antibody conjugate solution could be stored at 4 °C for two months and kept away from light. For longer storage, the HRP-antibody conjugates could be lyophilized and stored at ≤ –20 °C.
References
Authors: Tresca JP, Ricoux R, Pontet M, Engler R.
Journal: Ann Biol Clin (Paris) (1995): 227
Authors: Presentini R, Terrana B.
Journal: J Immunoassay (1995): 309
Authors: Strakova Z, Barancik M, Lukacova D, Angyal R, Slosarcikova L, Horakova K.
Journal: Gen Physiol Biophys (1991): 63
Authors: Tijssen P, Kurstak E.
Journal: Anal Biochem (1984): 451
Authors: Boorsma DM, Cuello AC, van Leeuwen FW.
Journal: J Histochem Cytochem (1982): 1211