Buccutite™ Poly-HRP Antibody Conjugation Kit
Example protocol
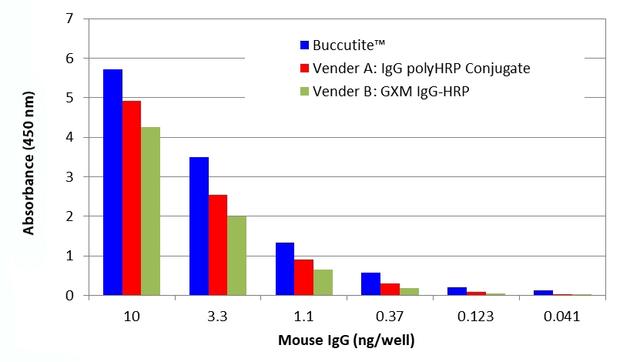
AT A GLANCE
Add 5 µL of Reaction Buffer (Component C) into antibody (50 µL)
- Add the antibody solution into the Buccutite™ MTA vial (Component B)
- Incubate at room temperature for 30 minutes
- Mix with 200 µL of Buccutite™ FOL-Activated Poly-HRP (Component A)
- Incubate at room temperature for 60 minutes
Warm up all the components to room temperature and centrifuge the vials briefly before opening. Immediately prepare the required solutions before starting your conjugation. The following SOP is an example for labeling 50 µg goat anti-mouse IgG antibody.
PREPARATION OF WORKING SOLUTION
For labeling 50 µg antibody (assuming the target antibody concentration is 1 mg/mL), mix 5 µL (5% of the total reaction volume) of Reaction Buffer (Component C) with 50 µL of the target antibody solution.
Note: If you have a different concentration, adjust the antibody volume accordingly to make ~50 µg antibody/50 µL available for your labeling reaction.
Note: The antibody should be dissolved in 1X phosphate buffered saline (PBS), pH 7.2-7.4. If the antibody is dissolved in glycine buffer, it must be dialyzed against 1X PBS, pH 7.2-7.4, or use 10KD Filter (Cat. # 60502 from AAT Bioquest) to remove free amines or ammonium salts (such as ammonium sulfate and ammonium acetate).
Note: Impure antibodies or antibodies stabilized with bovine serum albumin (BSA) or gelatin will not be labeled well.
Note: The antibody –Buccutite™ MTA reaction efficiency is significantly reduced if the antibody concentration is less than 1 mg/mL. For optimal labeling efficiency, the final antibody concentration range of 1-10 mg/mL is recommended.
SAMPLE EXPERIMENTAL PROTOCOL
- Add the antibody working solution directly into the vial of Buccutite ™ MTA (Component B), and mix them well by repeatedly pipetting for a few times or vortex the vial for a few seconds.
- Incubate the antibody-Buccutite ™ MTA reaction mixture at room temperature for 30 minutes.
Note The antibody-Buccutite™ MTA reaction mixture can be rotated or shaken for longer time if desired.
- Add antibody-Buccutite™ MTA reaction mixture to the vial of Buccutite™ FOL-Activated Poly-HRP (Component A), mix well and incubate the mixture at room temperature for 60 minutes.
Note The antibody poly-HRP reaction mixture can be incubated for longer time if desired. - The antibody-poly-HRP conjugate is now ready to use.
Note For immediate use, the antibody-poly-HRP conjugate needs to be diluted with the buffer of your choice. - The concentration of the conjugate can be calculated as follows: Antibody Concentration (µg/µL) = 50 µg (total amount of antibody)/(50 µL + 5 µL+ 200 µL) = 0.196 µg/µL
The antibody conjugate-polyHRP conjugate should be stored in the presence of a carrier antibody (e.g., 0.1% bovine serum albumin) at 4 °C and kept away from light for upto two months.
References
Authors: Sharafeldin, Mohamed and Chen, Tianqi and Ozkaya, Gulsum Ucak and Choudhary, Dharamainder and Molinolo, Alfredo A and Gutkind, J Silvio and Rusling, James F
Journal: Biosensors & bioelectronics (2021): 112681
Authors: Jones, Abby L and Dhanapala, Lasangi and Baldo, Thaísa A and Sharafeldin, Mohamed and Krause, Colleen E and Shen, Min and Moghaddam, Shirin and Faria, Ronaldo C and Dey, Dipak K and Watson, R William and Andrawis, Ramez and Lee, Norman H and Rusling, James F
Journal: Analytical chemistry (2020)
Authors: Hira, Vashendriya V V and de Jong, Annique Loncq and Ferro, Klea and Khurshed, Mohammed and Molenaar, Remco J and Van Noorden, Cornelis J F
Journal: Acta histochemica (2019): 125-134
Authors: Masoud, Rokia and Ibrahiem, Afaf and Tantawy, Dina and Eldosoky, Ibrahim
Journal: Annals of diagnostic pathology (2019): 64-68
Authors: Ben Ismail, Manel and de la Serna, Erica and Ruiz-Vega, Gisela and García-Berrocoso, Teresa and Montaner, Joan and Zourob, Mohammed and Othmane, Ali and Baldrich, Eva
Journal: Analytica chimica acta (2018): 144-154