Casein, TAMRA-conjugated
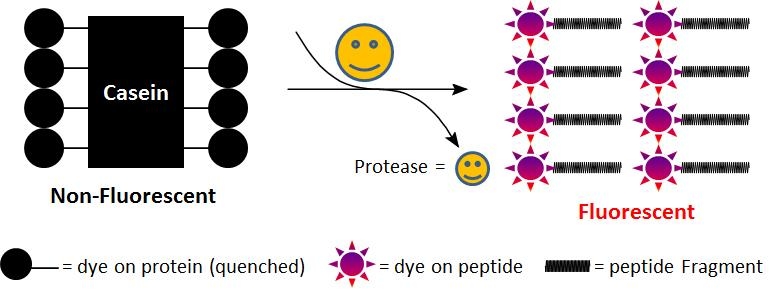
Casein is considered to be a generic substrate for a broad spectrum of proteases. As native casein this rhodaminated casein is hydrolyzed by many proteases, and widely used for fluorometric measurement of protease activity. In the intact substrate, casein is heavily labeled with TAMRA, resulting in significant fluorescence quenching. Protease-catalyzed hydrolysis relieves its quenching effect, yielding brightly orange fluorescent dye-labeled short peptides. The increase in fluorescence intensity is directly proportional to protease activity. Compared to FITC-labeled casein, this casein substrate has pH-independent fluorescence. This feature is more convenient for the assays that require low pH. We do not recommend that this conjugate be used for fluorescence polarization assay. For fluorescence polarization we can custom-make the lightly labeled fluorescein casein conjugate.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 13441 | 5 mg | Price |
Physical properties
| Molecular weight | N/A |
| Solvent | Water |
Spectral properties
| Correction factor (260 nm) | 0.32 |
| Correction factor (280 nm) | 0.178 |
| Extinction coefficient (cm -1 M -1) | 90000 |
| Excitation (nm) | 552 |
| Emission (nm) | 578 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 20, 2026

