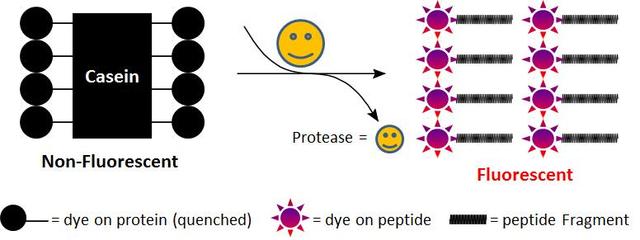
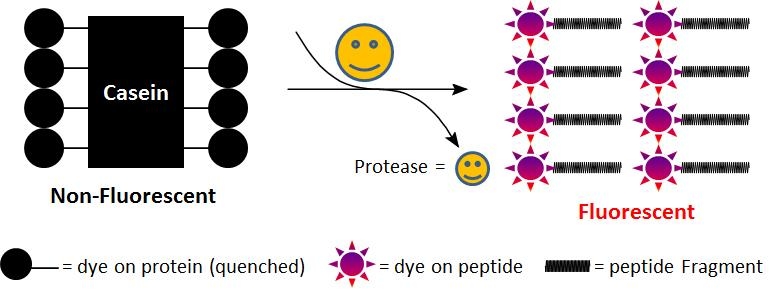
Casein, TAMRA-conjugated
Casein is considered to be a generic substrate for a broad spectrum of proteases. As native casein this rhodaminated casein is hydrolyzed by many proteases, and widely used for fluorometric measurement of protease activity. In the intact substrate, casein is heavily labeled with TAMRA, resulting in significant fluorescence quenching. Protease-catalyzed hydrolysis relieves its quenching effect, yielding brightly orange fluorescent dye-labeled short peptides. The increase in fluorescence intensity is directly proportional to protease activity. Compared to FITC-labeled casein, this casein substrate has pH-independent fluorescence. This feature is more convenient for the assays that require low pH. We do not recommend that this conjugate be used for fluorescence polarization assay. For fluorescence polarization we can custom-make the lightly labeled fluorescein casein conjugate.
Example protocol
PREPARATION OF STOCK SOLUTIONS
Unless otherwise noted, all unused stock solutions should be divided into single-use aliquots and stored at -20 °C after preparation. Avoid repeated freeze-thaw cycles.
Note Unused stock solution can be divided into single use aliquots and stored at -20 °C, and avoid exposure to light.
Casein, TAMRA-conjugated stock solution
Make a 2.5 - 5 mg/mL Casein, TAMRA-conjugated stock solution in PBS buffer.Note Unused stock solution can be divided into single use aliquots and stored at -20 °C, and avoid exposure to light.
PREPARATION OF WORKING SOLUTION
Casein TAMRA-conjugated working solution (2X)
Dilute the TAMRA-conjugated stock solution into 50 - 100 mM Tris buffer (pH 7.4) at 100 - 400 μg/mL. The 2X Assay working solution is designed for detecting the activity of chymotrypsin, trypsin, thermolysin, proteinase K, protease XIV, and human leukocyte elastase. For other proteases, please refer to Table 1 for the appropriate assay buffer formula. The optimum concentration of the assay working solution should be determined experimentally for individual proteases.SAMPLE EXPERIMENTAL PROTOCOL
Table 1. Appropriate assay buffer formula for Assay working solution.
| Protease | 1X Assay Buffer |
| Cathepsin D | 20 mM Sodium Citrate, pH 3.0 |
| Papain | 20 mM sodium acetate, 20 mM cysteine, 2 mM EDTA, pH 6.5 |
| PAE | 20 mM sodium phosphate, pH 8.0 |
| Pepsin | 10 mM HCl, pH 2.0 |
| Porcine pancreas elastase | 10 mM Tris-HCl, pH 8.8 |
| Subtilisin | 20 mM potassium phosphate buffer, pH 7.6, 150 mM NaCl |
- Mix equal volume of the trypsin standards or samples with 2X Assay working solution.
- Monitor the fluorescence increase at Ex/Em = 540/590 nm. a. For kinetic reading: Immediately start measuring fluorescence intensity continuously and record data every 5 minutes for 30 minutes. b. For end-point reading: Incubate the reaction at a desired temperature for 30 to 60 minutes, protected from light. Then measure the fluorescence intensity.
Spectrum
Product family
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Quantum yield | Correction Factor (280 nm) |
| Casein, FITC-conjugated | 491 | 516 | 73000 | 0.92 | 0.35 |
Citations
View all 3 citations: Citation Explorer
Liquid temperature measurement method in microchannels by using fluorescence polarization
Authors: Tatsumi, Kazuya and Hsu, Chi Hsuan and Suzuki, Atsushi and Nakabe, Kazuyoshi
Journal: Heat and Mass Transfer (2017): 1--10
Authors: Tatsumi, Kazuya and Hsu, Chi Hsuan and Suzuki, Atsushi and Nakabe, Kazuyoshi
Journal: Heat and Mass Transfer (2017): 1--10
Micro-scale temperature measurement method using fluorescence polarization
Authors: Tatsumi, K and Hsu, CH and Suzuki, A and Nakabe, K
Journal: (2016): 032097
Authors: Tatsumi, K and Hsu, CH and Suzuki, A and Nakabe, K
Journal: (2016): 032097
Microscopic Fluid Temperature Measurements Using Fluorescence Polarization Method
Authors: Tatsumi, Kazuya and Tozaki, Akihisa and Nakabe, Kazuyoshi
Journal: (2011): T10167--T10167
Authors: Tatsumi, Kazuya and Tozaki, Akihisa and Nakabe, Kazuyoshi
Journal: (2011): T10167--T10167
References
View all 30 references: Citation Explorer
Transient kinetic experiments demonstrate the existence of a unique catalytic enzyme form in the peptide-stimulated ATPase mechanism of Escherichia coli Lon protease
Authors: Vineyard D, Zhang X, Lee I.
Journal: Biochemistry (2006): 11432
Authors: Vineyard D, Zhang X, Lee I.
Journal: Biochemistry (2006): 11432
Highly stable glycosylated serine protease from the medicinal plant Euphorbia milii
Authors: Yadav SC, P and e M, Jagannadham MV.
Journal: Phytochemistry (2006): 1414
Authors: Yadav SC, P and e M, Jagannadham MV.
Journal: Phytochemistry (2006): 1414
Effects of Pseudomonas fluorescens M3/6 bacterial protease on plasmin system and plasminogen activation
Authors: Frohbieter KA, Ismail B, Nielsen SS, Hayes KD.
Journal: J Dairy Sci (2005): 3392
Authors: Frohbieter KA, Ismail B, Nielsen SS, Hayes KD.
Journal: J Dairy Sci (2005): 3392
Fibrillar amyloid beta-protein inhibits the activity of high molecular weight brain protease and trypsin
Authors: Chauhan V, Sheikh AM, Chauhan A, Spivack WD, Fenko MD, Malik MN.
Journal: J Alzheimers Dis (2005): 37
Authors: Chauhan V, Sheikh AM, Chauhan A, Spivack WD, Fenko MD, Malik MN.
Journal: J Alzheimers Dis (2005): 37
Characterization of a novel and specific inhibitor for the pro-apoptotic protease Omi/HtrA2
Authors: Cilenti L, Lee Y, Hess S, Srinivasula S, Park KM, Junqueira D, Davis H, Bonventre JV, Alnemri ES, Zervos AS.
Journal: J Biol Chem (2003): 11489
Authors: Cilenti L, Lee Y, Hess S, Srinivasula S, Park KM, Junqueira D, Davis H, Bonventre JV, Alnemri ES, Zervos AS.
Journal: J Biol Chem (2003): 11489
Page updated on August 28, 2025