Cell Meter™ Flow Cytometric Calcium Assay Kit
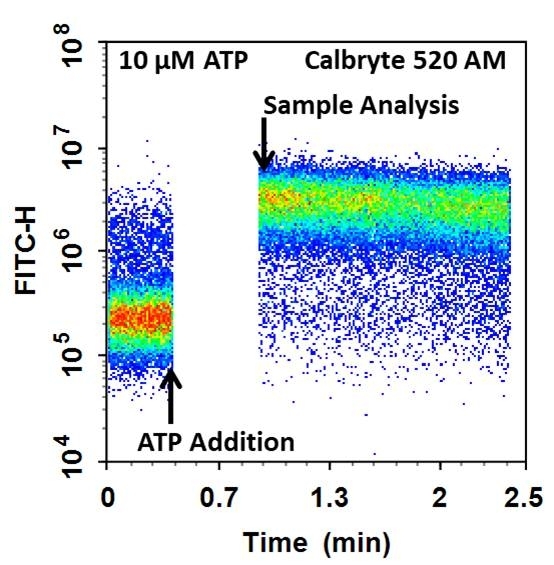
Cell Meter™ Flow Cytometric Calcium Assay Kit provides fluorescence-based assays for detecting intracellular calcium mobilization using a flow cytometer. It can be used for kinetic reading or for endpoint reading. After loading the Calbryte™ 520 AM dye into cells of interest, simply wash the cells and add the calcium flux agonist, one can then read the sample via a flow cytometer using kinetic reading mode or endpoint reading mode. Calbryte™ 520 AM can cross cell membrane passively by diffusion. Once inside the cells, the lipophilic blocking groups of Calbryte™ 520 AM are cleaved by esterase, resulting in a negatively charged fluorescent dye that stays inside cells. Its fluorescence is greatly enhanced upon binding to calcium. When cells expressing GPCR of interest are stimulated with an agonist, the receptor signals the release of intracellular calcium, which significantly increases the fluorescence of Calbryte™ 520. The Cell Meter™ Flow Cytometric Calcium Assay Kit can be used for monitoring cellular calcium flux as well as cell sorting.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 36310 | 100 Tests | Price |
Spectral properties
| Excitation (nm) | 493 |
| Emission (nm) | 515 |
| Quantum yield | 0.75 1 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 488 nm laser |
| Emission | 530/30 nm filter |
| Instrument specification(s) | FITC channel |
| Other instruments | ArrayScan, FDSS, FlexStation, IN Cell Analyzer, NOVOStar, ViewLux |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 20, 2026

