Portelite™ Fluorimetric RNA Quantification Kit
20-1000 ng Broad Range
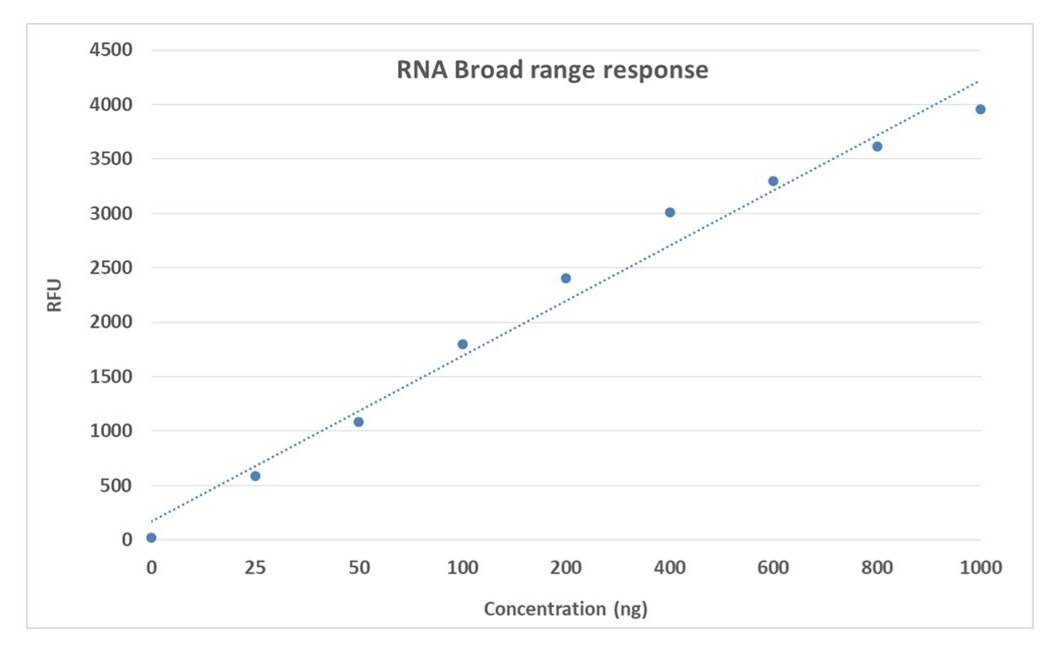
Portelite™ Fluorimetric RNA Quantification Assay Kit accurately measures RNA across a broad range for research applications.
- Broad range detection: Measures RNA in the 20–1000 ng range with high accuracy using a fluorescence-based assay
- High specificity: Selectively detects RNA with minimal interference from DNA, proteins, or other contaminants
- Applications: Suitable for RT-qPCR, RNA-seq, cDNA synthesis, and other RNA-based studies
- Comparable alternative: Provides a performance advantage over traditional absorbance-based RNA quantification kits with reduced interference and higher accuracy

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 17697 | 100 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
Instrument settings
| Qubit Fluorometer | |
| Excitation | 635 nm |
| Emission | 665-720 nm |
| Instrument specification(s) | 0.2 mL PCR vial |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on March 9, 2026
