ReadiUse™ 6-Color Human TBNK Antibody Kit
Dry Reagent Format
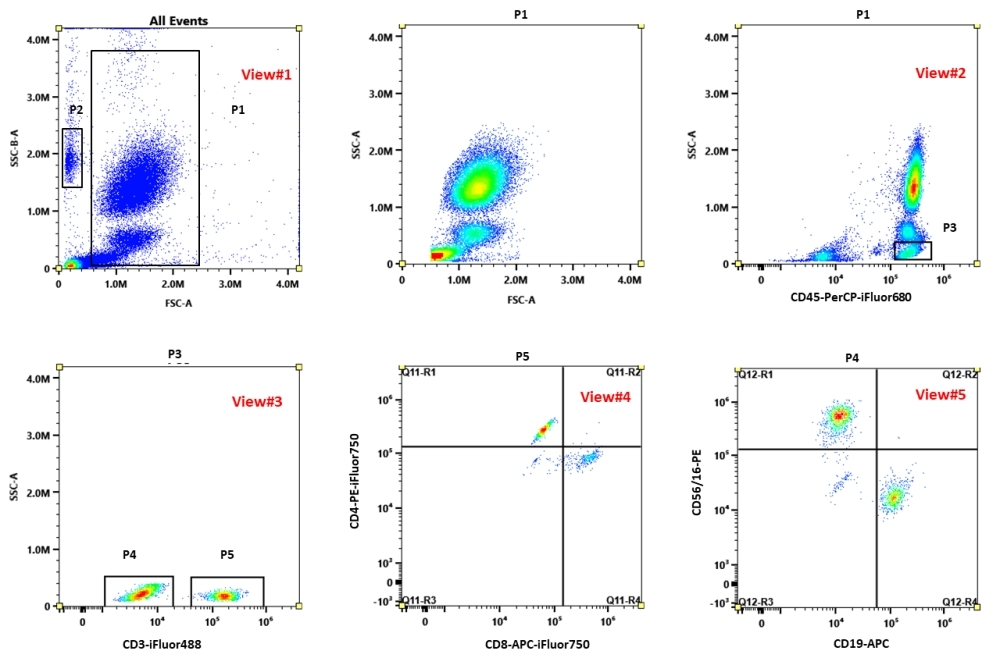
The ReadiUse™ 6-Color Human TBNK Antibody Kit for flow cytometry utilizes dried multicolor beads to delineate principal lymphocyte subsets - CD4+ T cells, CD8+ T cells, B cells, and NK cells – in whole blood specimens. Each TBNK dried reagent contains a cocktail of fluorescently-labeled monoclonal antibodies directed against human CD3, CD4, CD8, CD16, C19, CD45, and CD56. The kit is designed to be used in a “lyse/no-wash” format for quick and easy sample preparation, with less inconsistencies. Also included in this kit is a viability stain to identify dead cells and a precise number of fluorescent counting beads to determine absolute lymphocyte subset cell concentrations.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 90010 | 5 Tests | Price | |
| 90011 | 25 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 23, 2026
