Xite™ Red beta-D-galactopyranoside
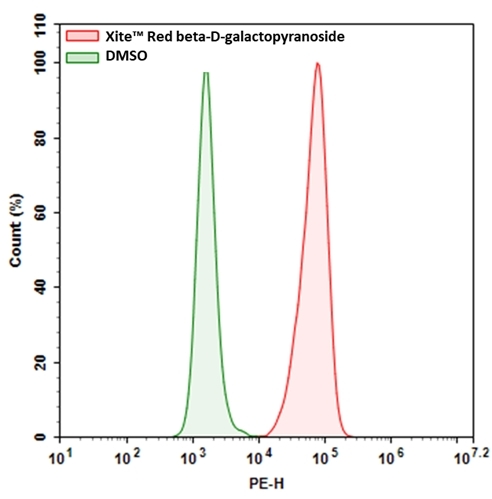
Xite™ Red beta-D-galactopyranoside provides a simple and sensitive tool to detect beta-galactosidase (β-gal) activity. Compared to the existing red beta-galactosidase substrates (e.g., the commonly used resorufin beta-D-galactopyranoside), it has much better cell permeability. Xite™ Red beta-D-galactopyranoside provides a simple and sensitive tool to detect beta-galactosidase activity. Xite™ Red beta-D-galactopyranoside might be used as a simple tool for measuring cellular senescence in cells since β-gal has been identified as a reliable marker for cellular senescence. Xite™ Red beta-D-galactopyranoside enters readily cells where it gets cleaved by β-gal, producing Xite™ Red, a strongly fluorescent product. The strongly fluorescent Xite™ Red is well retained in cells, making it easy to be detected with a flow cytometer and fluorescence microscope. In addition, Xite™ Red beta-D-galactopyranoside is fixable. The red fluorescence generated by Xite™ Red beta-D-galactopyranoside can be readily combined with other color fluorescent probes such as DAPI or GFP for multicolor fluorescence analysis.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 14035 | 1 mg | Price |
Physical properties
| Molecular weight | 591.63 |
| Solvent | DMSO |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Instrument settings
| Flow cytometer | |
| Excitation | 488 nm laser |
| Emission | 575/26 nm filter |
| Instrument specification(s) | PE channel |
| Fluorescence microscope | |
| Excitation | Cy3/TRITC filter set |
| Emission | Cy3/TRITC filter set |
| Recommended plate | Black wall/clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 12, 2026
