MMP-3 Green™ substrate solution
Ordering information
| Price | |
| Catalog Number | |
| Unit Size | |
| Quantity |
Additional ordering information
| Telephone | 1-800-990-8053 |
| Fax | 1-800-609-2943 |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Shipping | Standard overnight for United States, inquire for international |
Physical properties
| Molecular weight | ~2200 |
| Solvent | DMSO |
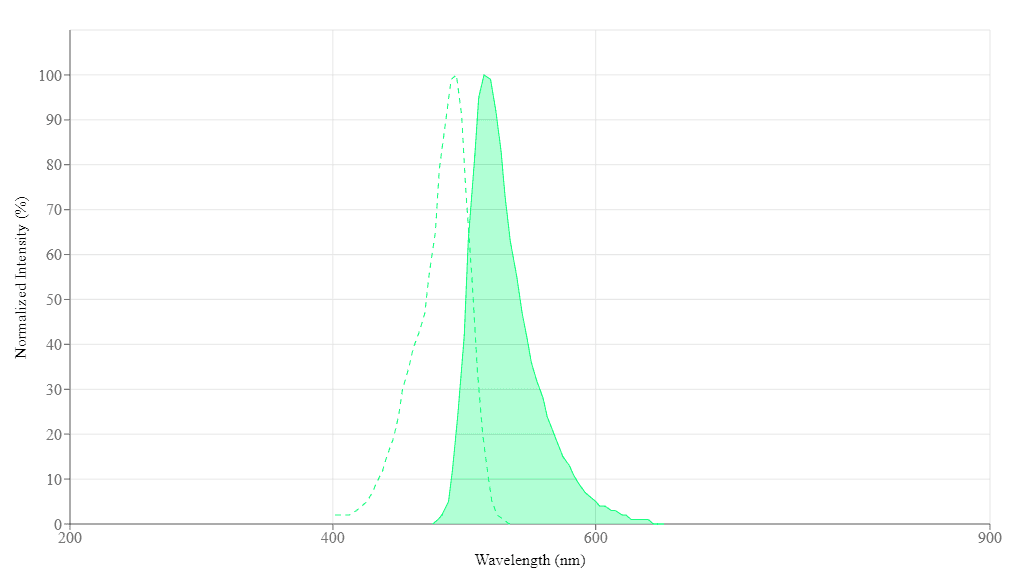
Spectral properties
| Excitation (nm) | 494 |
| Emission (nm) | 515 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Example protocol
AT A GLANCE
AT A GLANCE
Protocol Summary
- Add appropriate controls, or test samples (50 µL)
- Pre-incubate for 10 - 15 minutes
- Add MMP-3 Green™ substrate working solution (50 µL)
- Skip incubation for kinetic reading or incubate 30 to 60 minutes for end point reading
- Monitor fluorescence intensity at Ex/Em = 490/525 nm
Important
Thaw the solution at room temperature before starting the experiment. Prepare MMP-3 containing biological samples as desired.PREPARATION OF WORKING SOLUTION
1. MMP-3 Green™ Substrate working solution
Add 50 μL of MMP-3 Green™ Substrate Solution into 5 mL of buffer of your choice to make a total volume of 5.05 mL. Note: Tris buffer can be used for the assay.2. MMP-3 dilutions
Dilute MMP-3 to an appropriate concentration in buffer of your choice if purified MMP-3 is used. Note: MMP-3 needs to be activated before use. Avoid vigorous vortexing of the enzyme.3. Inhibitors and compounds dilution
Make an appropriate concentration of known MMP-3 inhibitors and test compounds dilutions as desired if screening MMP-3 inhibitors.SAMPLE EXPERIMENTAL PROTOCOL
- Prepare MMP-3 containing biological samples as desired.
- Activate pro-MMP-3 as per protocol. Note: Incubate the MMP-3 containing-samples or purified MMP-3 with equal volume of 2 mM APMA working solution (2X) at 37 °C for 24 hours. Activate MMP-3 immediately before the experiment.
- Prepare controls and test samples (TS) according to the layout provided in Tables 1 and 2. For a 384-well plate, use 20 µL of reagent per well instead of 50 µL.
- Pre-incubate the plate at a desired temperature for the enzyme reaction (e.g. 25 °C or 37 °C) for 10 - 15 minutes if you are screening MMP-3 inhibitors.
- Add 50 µL (96-well) or 20 µL (384-well) of MMP-3 Green™ substrate working solution to the sample and control wells of the assay plate. Mix the reagents well.
- Monitor the fluorescence intensity with a fluorescence plate reader at Ex/Em = 490/525 nm. For kinetic reading: Immediately start measuring fluorescence intensity and continuously record data every 5 minutes for 30 to 60 minutes. For end-point reading: Incubate the reaction at room temperature for 30 to 60 minutes, kept from light if possible. Mix the reagents well, and then measure the fluorescence intensity.
| SC | SC | ... | ... |
| IC | IC | ||
| VC | VC | ||
| TC | TC | ||
| TS | TS | ||
| ... | ... | ||
| ... | ... | ||
| Well | Volume | Reagent |
| SC | 50 µL | Buffer of your choice |
| IC | 50 µL | MMP-3 dilution and known MMP-3 inhibitor |
| VC | 50 µL | MMP-3 dilution and vehicle used to deliver test compound |
| TC | 50 µL | MMP-3 containing buffer and test compound |
| TS | 50 µL | MMP-3 dilution with test compound |
Application notes
A New Protein Crosslinking Method for Labeling and Modifying Antibodies
Abbreviation of Common Chemical Compounds Related to Peptides
An Increase in Plasma Homovanillic Acid with Cocoa Extract Consumption Is Associated with the Alleviation of Depressive Symptoms in Overweight or Obese Adults
Attenuation of lysyl oxidase and collagen gene expression in keratoconus patient corneal epithelium corresponds to disease severity
β-adrenoceptors are upregulated in human melanoma and their activation releases pro-tumorigenic cytokines and metalloproteases in melanoma cell lines
Abbreviation of Common Chemical Compounds Related to Peptides
An Increase in Plasma Homovanillic Acid with Cocoa Extract Consumption Is Associated with the Alleviation of Depressive Symptoms in Overweight or Obese Adults
Attenuation of lysyl oxidase and collagen gene expression in keratoconus patient corneal epithelium corresponds to disease severity
β-adrenoceptors are upregulated in human melanoma and their activation releases pro-tumorigenic cytokines and metalloproteases in melanoma cell lines