A New Protein Crosslinking Method for Labeling and Modifying Antibodies
by Haitao Guo, Jinfang Liao, Xing Han, Zhenjun Diwu
The covalent conjugation of a large biomolecule to another macromolecule, such as an antibody to an enzyme, or an enzyme to DNA is an important task for researchers. There are a few methods available for crosslinking two biomolecules. One common method is to use a small crosslinker (such as Sulfo-SMCC). SMCC has an amine-reactive NHS ester group at one end to react with free amines (-NH2 ) of a protein, and a sulfhydryl-reactive maleimide group at the other end to react with a thiol group (-SH) of biomolecule to be conjugated. SMCC-modified linker on a protein is extremely unstable and often self-reactive since proteins often contain both free amines and thiol groups, resulting in a significant amount of homo-crosslinking. In addition, the quantitation of the number of maleimide groups on a protein is quite tedious, and it is difficult to set the suitable molar ratio of two biomolecules to be linked in the reaction for the desirable function and coupling efficiency.
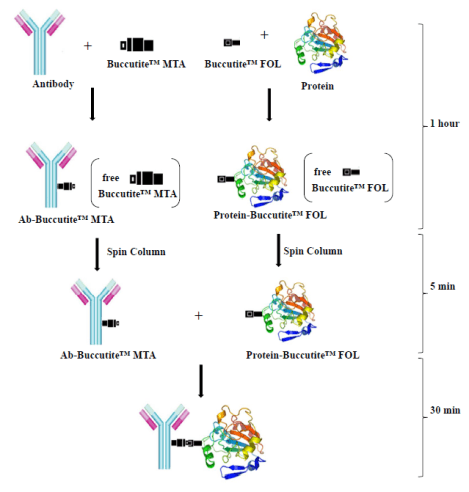
A convenient and effective crosslinking method has been developed to link two biomolecules with a high conjugation yield. This method uses two unique crosslinkers (Buccutite™ MTA and Buccutite™ FOL). The crosslinkers of MTA and FOL on proteins are easy to control and quantify. Following a desalting column to remove free linkers, and the Buccutite™MTA activated antibody and Buccutite™ FOL modified protein readily react upon mixing under a mild condition. The purification is essentially unnecessary, and there is no homo-crosslinking of each biomolecule in the reaction.
Original created on May 26, 2015, last updated on May 26, 2015
Tagged under:
Introduction
The covalent conjugation of a large biomolecule to another macromolecule, such as an antibody to an enzyme, or an enzyme to DNA is an important task for researchers. There are a few methods available for crosslinking two biomolecules. One common method is to use a small crosslinker (such as Sulfo-SMCC). SMCC has an amine-reactive NHS ester group at one end to react with free amines (-NH2 ) of a protein, and a sulfhydryl-reactive maleimide group at the other end to react with a thiol group (-SH) of biomolecule to be conjugated. SMCC-modified linker on a protein is extremely unstable and often self-reactive since proteins often contain both free amines and thiol groups, resulting in a significant amount of homo-crosslinking. In addition, the quantitation of the number of maleimide groups on a protein is quite tedious, and it is difficult to set the suitable molar ratio of two biomolecules to be linked in the reaction for the desirable function and coupling efficiency.
A convenient and effective crosslinking method has been developed to link two biomolecules with a high conjugation yield. This method uses two unique crosslinkers (Buccutite™ MTA and Buccutite™ FOL). The crosslinkers of MTA and FOL on proteins are easy to control and quantify. Following a desalting column to remove free linkers, and the Buccutite™MTA activated antibody and Buccutite™ FOL modified protein readily react upon mixing under a mild condition. The purification is essentially unnecessary, and there is no homo-crosslinking of each biomolecule in the reaction.
Materials and Methods
- Antibodies and Enzymes: Goat-Anti-Mouse IgG and mouse IgG are from Jackson Immuno Research Laboratories; HRP is from Calzyme, Tubulin mouse mAb is from Life Technologies.
- Purification Columns: All purification columns are Bio-Spin size exclusion columns from Bio-Rad.
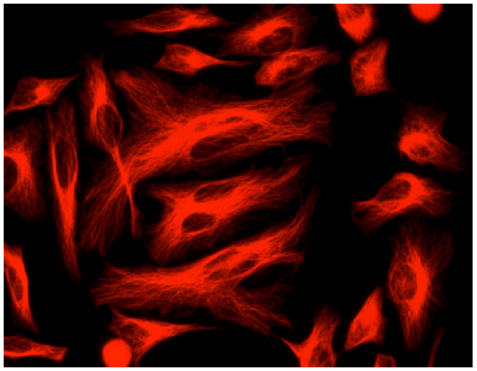
- Cells Imaging: HeLa cells are fixed with 4% formaldehyde, and stained with Mouse Anti-tubulin, and then stained with the HRP-Goat–Anti-Mouse IgG conjugate. Images were taken with Olympus IX71 fluorescence microscope.
Conjugation Principles
- Goat-Anti-Mouse IgG was activated with MTA , and tag protein (APC or PE) was modified with FOL (1hour).
- The activated IgG and modified tag protein was purified with a spin column (5min).
- Mix the activated antibody and modified tag protein (30 min).
- Stain cells with the desired conjugate without further purification.
Results
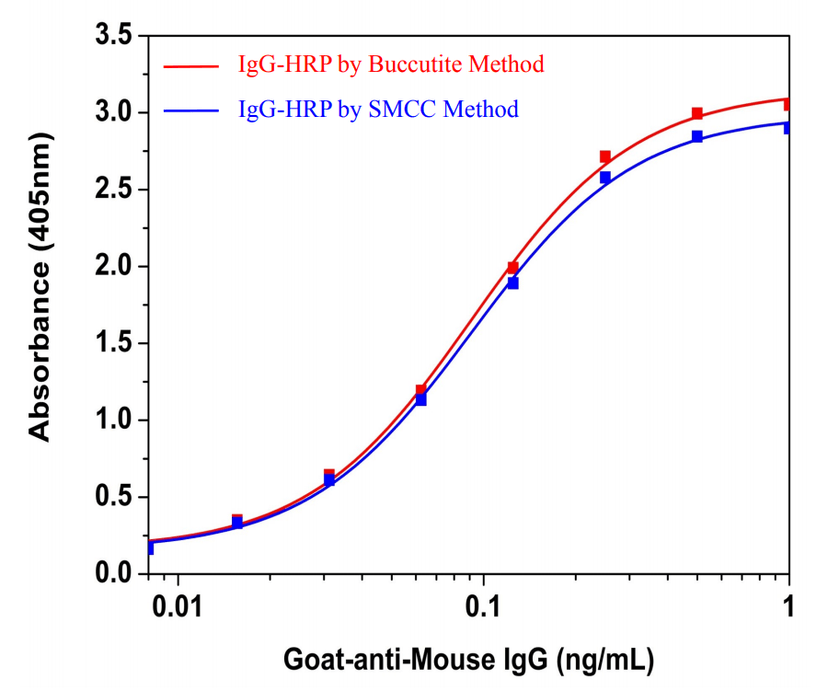
Buccutite™ HRP-IgG Conjugate for ELISA Assays
Direct ELISA curves were generated using the HRP- goat-anti-mouse IgG conjugate prepared with Buccutite™ crosslinking technology (in red) and with SMCC method (in blue). Mouse monoclonal antibody (100 ng) was coated on a 96-well plate, GAM IgG-HRP conjugate was diluted in a 2-fold dilution series using the standard method. ABTS substrate solution (#11001, AAT Bioquest) was used to detect the immobilized mouse IgG with 30 min incubation and read at 405 nm.
Buccutite™ IgG-RPE Conjugate for cell imaging
Summary
- Linkers are highly stable. The Buccutite™ linker-activated macromolecules are very stable, can be stored at 4ºC for more than 24 months.
- Conjugation conditions are extremely mild and robust. Buccutite™ conjugations can be run in a broad range of pH, concentration and temperature. No catalyst is required.
- High yield: Buccutite™ conjugation can be finished within 1 hour. Buccutite™ conjugation gives much higher yield than other existing methods under the same conditions. The conjugation can be run at extremely low concentration.
Original created on May 26, 2015, last updated on May 26, 2015
Tagged under: