A New Robust No-Wash FLIPR Calcium Assay Kit for Screening GPCR and Calcium Channel Targets
by Chunmei Wei, Jianjun He, Jinfang Liao, Zhenjun Diwu
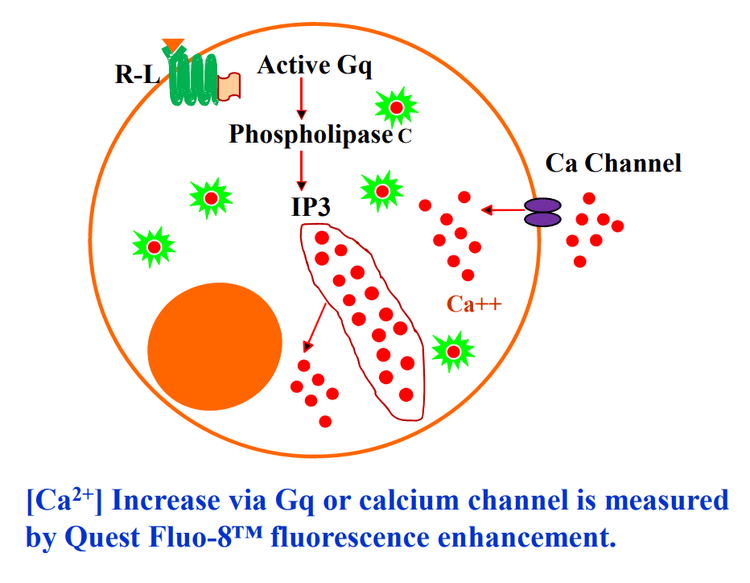
Calcium flux assays are preferred methods in drug discovery for screening F protein coupled recepters (GPCRs) Screen Quest™ Fluo-8 No Wash Calcium Assay Kits provides homogeneous fluorescence-based assays for detecting intracellular calcium mobilization across a broad spectrum of biological targets. The characteristics of its long wavelength, high sensitivity and 200 times fluorescence enhancement (when it forms a complex with calcium) make Fluo-8 NW an ideal indicator for measurement of cellular calcium.
The signal intensity and well-to-well uniformity of Fluo-8 NW is compared with Fluo-3 AM, Fluo-4 NW yields the brightest signal, more the 2 fold brighter than Fluo-4 AM, and 4 times brighter than Fluo-3 AM. The Screen Quest™ Fluo-8 NW Calcium Assay Kits provide an optimized assay method for monitoring GPCRs and calcium channels. Unlike Fluo-3 AM and Fluo-4 AM, both of which require 37°C for optimal cell loading, the Screen Quest™ Fluo-8 NW requires a less temperature-dependent cell loading property, giving similar results either at room temperature or 37°C. This characteristic makes the Screen Quest™ Fluo-8 NW Calcium Assay Kits more robust for HTS applications.
The Screen Quest™ Fluo-8 NW Calcium Assay kit has been optimized for broad range of instruments to give maximum performance with GPCR and calcium channel tagets.
The Screen Quest™ Fluo-8 NW Calcium Assay Kit provides an optimized assay method for monitoring G-protein-coupled receptors (GPCRs) and calcium channels. Its ability to generate a very large signal from the weak and robust assay systems enables researchers to have multiple assay chemistries for different receptor and calcium channel targets.
Original created on April 13, 2008, last updated on April 13, 2008
Tagged under:
Introduction
Calcium flux assays are preferred methods in drug discovery for screening F protein coupled recepters (GPCRs) Screen Quest™ Fluo-8 No Wash Calcium Assay Kits provides homogeneous fluorescence-based assays for detecting intracellular calcium mobilization across a broad spectrum of biological targets. The characteristics of its long wavelength, high sensitivity and 200 times fluorescence enhancement (when it forms a complex with calcium) make Fluo-8 NW an ideal indicator for measurement of cellular calcium.
The signal intensity and well-to-well uniformity of Fluo-8 NW is compared with Fluo-3 AM, Fluo-4 NW yields the brightest signal, more the 2 fold brighter than Fluo-4 AM, and 4 times brighter than Fluo-3 AM. The Screen Quest™ Fluo-8 NW Calcium Assay Kits provide an optimized assay method for monitoring GPCRs and calcium channels. Unlike Fluo-3 AM and Fluo-4 AM, both of which require 37°C for optimal cell loading, the Screen Quest™ Fluo-8 NW requires a less temperature-dependent cell loading property, giving similar results either at room temperature or 37°C. This characteristic makes the Screen Quest™ Fluo-8 NW Calcium Assay Kits more robust for HTS applications.
Materials and Methods
- CHO-M1 or HEK cells were plated at 96-well (for NOVOstar) or 384-well black wall/clear bottom costar plate (for FLIPR) at 37°C incubator for overnight.
- Take growth medium off (if cells were plating in 0.51% FBS, skip this step), incubate the cells with Fluo-3 AM, Fluo-4 AM or Fluo-8 NW at room temp for 1-2 hr (or at 37°C for 30 min, then at room temp. for 30 min).
- For wash experiments: wash cells with HHBS buffer twice, then replace with HHBS buffer.
- For No wash experiments: run the experiments directly.
- Run calcium efflux experiments on NOVOstar or FLIPR
Results
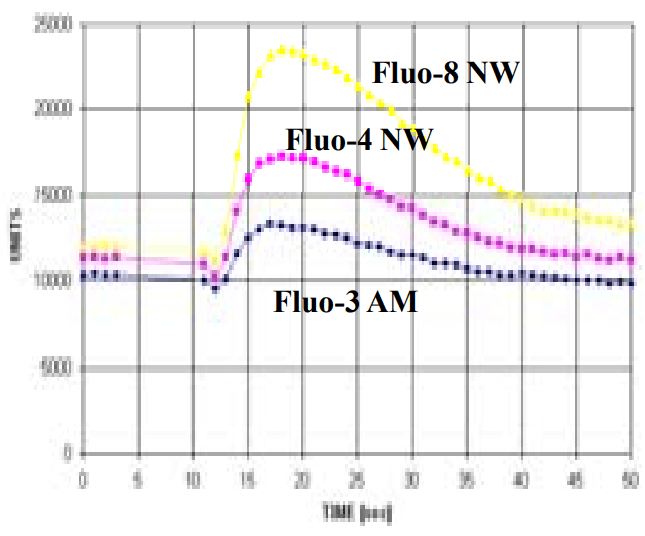
U2OS cells were seeded overnight at 40,000 cells per 100 µL per well in a 96-well black wall/clear bottom costar plate. The growth medium was removed, and the cells were incubated with 100 µL of 4 µM Fluo-3 AM, Fluo-4 AM or Quest Fluo-8™ AM in HHBS at 37°C for 1 hour. The cells were washed twice with 200 µL HHBS, then imaged with a fluorescence microscope (Olympus IX71) using FITC channel.
NOVOStar 96-well data
Comparisons of Screen Quest™ Fluo-8 NW Calcium Assay Kit, Fluo-4 NW, and Fluo-3 AM in HEK-293 cells were seed overnight in 40,000 cells per 100 µL per well in a 96-well black wall/clear bottom costar plate. The growth medium was removed, and the cells were incubated with 100 µL of Screen Quest™ Fluo-8 NW, Fluo-4 NW, or Flu-3 AM, for 1 hr at 37°C. 30 µM of Carbachol (50 µL/well) was added by NOVOstar (BMG Labtech).
NOVOStar 96-well data
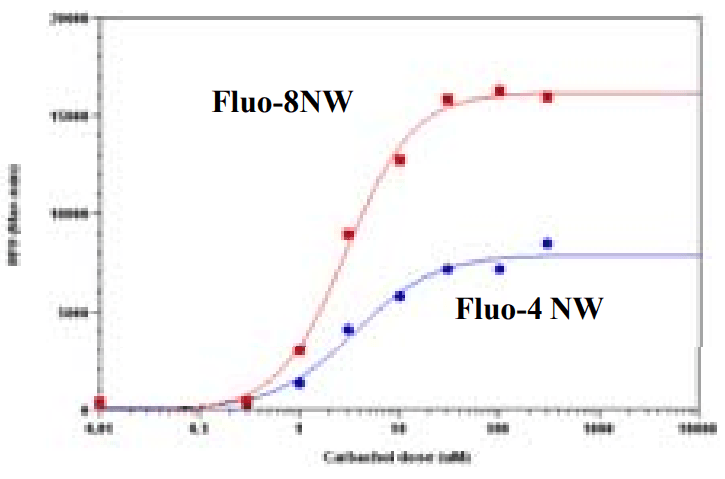
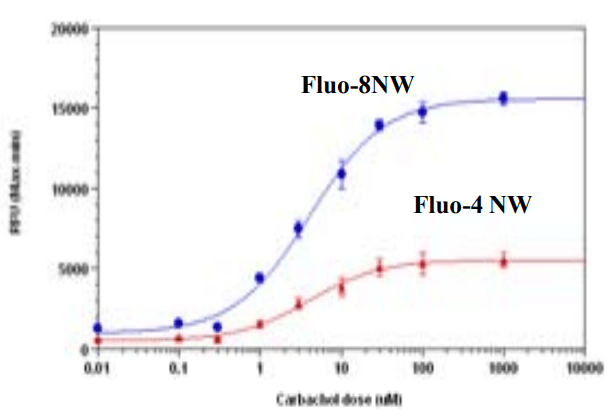
Carbachol Dose Response in HEK-293. HEK-293 cells were seed overnight in 40,000 per 25 µL per well in growth medium with 1% FBS in a 96-well black wall/clear bottom costar plate. The cells were incubated with 100 µL of the Screen Quest™ Fluo-8 NW Calcium Assay kit, or Fluo-4 NW kit (based on manufacture's protocol) for 1 hour at room temperature. Cabbachol (50 µL/well) was added by NOVOstar (BMG labtech) to achieve the final indicated concentration. The EC50 is about µM which is similar as reported.
FLIPR 384-well data
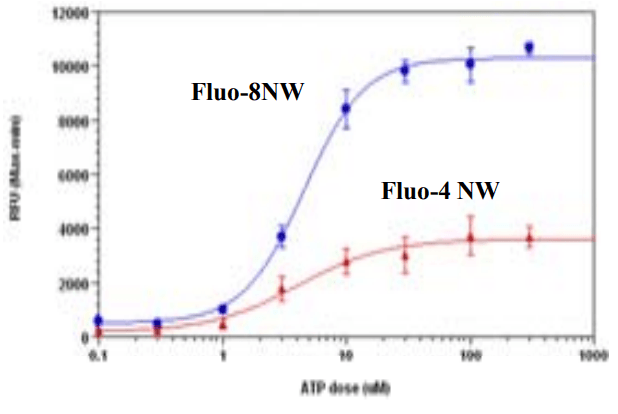
ATP Dose Response in HEK-293. HEK-293 cells were seeded overnight in 10,000 cells per 25 µL per well in a 384-well black wall/clear bottom costar plate. The growth medium was removed and the half of the plate was incubated with 25 µL of the Fluo-8 NW Calcium assay kit, and the other half of the plate were incubated with Fluo-4 NW kit (based on manufacture's protocol) for 1 hour at room temperature. ATP (12.5 µL/well) was added by FLIPR 384 to achieve the final indicated concentration. The EC50 is about 4 µM which is similar as reported.
FLIPR 384-well data
Carbachol Dose Response in HEK-293. HEK -293 cells were seeded overnight in 10,000 cells per 25 µL per wll in a 384-well black wall/clear bottom costar plate. The growth medium was removed and the half of the plate was incubated with 25 µL of the screen Quest™ Fluo-8 NW Calcium Assay kit, and the other half of the plate were incubated with Fluo-4 NW kit for 1 hour at room remperature. Carbachol (12.5 µL/well) was added by FLIPR 384 to achieve the final indicated concentration. The EC50 is about 4 µM which is similar as reported.
NOVOStar 96-well data
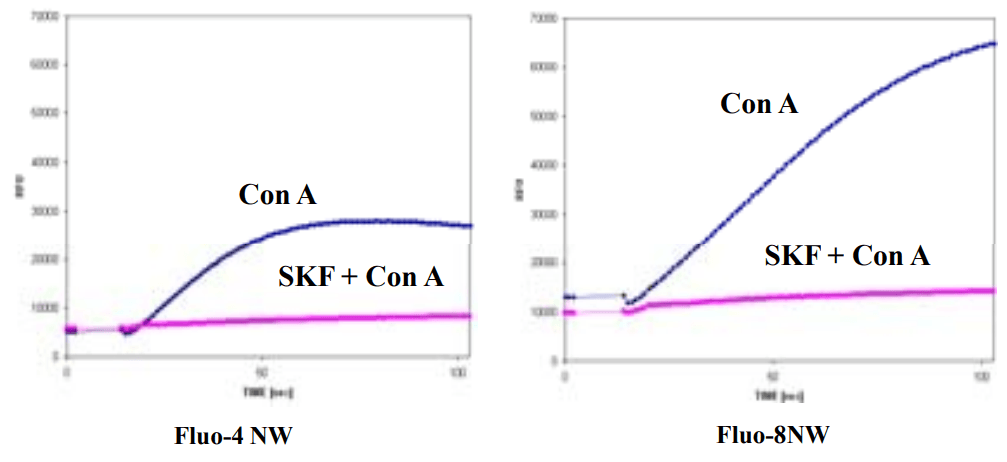
Comparisons of Screen Quest™ Fluo-8 NW Calcium Assay Kit, Fluo-4 NW on Concanavalin A induced Ca entry (capacitative calcium entry (CCE)) in JurKat cells. Jurkat cells were suspended at 4X106 cells per ml in calcium-free HHBS buffer at 2X105 cells/well/100 µL at a 96-well black wall/clear bottom costar plate for 1 hr at37oC, 5% CO2 incubator. At the end of the 10 min incubation, the channel opener Concanavalin A at 1 µg/mL with or without the channel inhibitor SKF96365 at 30 µM were added into the cells. 25 L/well of HBSS with additional 24 mM (5X) Calcium (so final in well concentration of calcium is 5 mM) was added by NOVOstar (BMG Labtech).
Summary
The Screen Quest™ Fluo-8 NW Calcium Assay kit has been optimized for broad range of instruments to give maximum performance with GPCR and calcium channel tagets.
- Brighter: Enable calcium assays with demanding cell lines and receptors;
- 1536 Well-friendly: best Ca2+ indicator for 1536-well Ca2+ assays;
- Less temperature-Sensitive: 37°C & RT loadings generate similar results;
- Larger assay window: Capable of assaying the challenging cell lines and receptors.
Conclusions
The Screen Quest™ Fluo-8 NW Calcium Assay Kit provides an optimized assay method for monitoring G-protein-coupled receptors (GPCRs) and calcium channels. Its ability to generate a very large signal from the weak and robust assay systems enables researchers to have multiple assay chemistries for different receptor and calcium channel targets.
Original created on April 13, 2008, last updated on April 13, 2008
Tagged under: