Amplite® Colorimetric Acetylcholinesterase Assay Kit
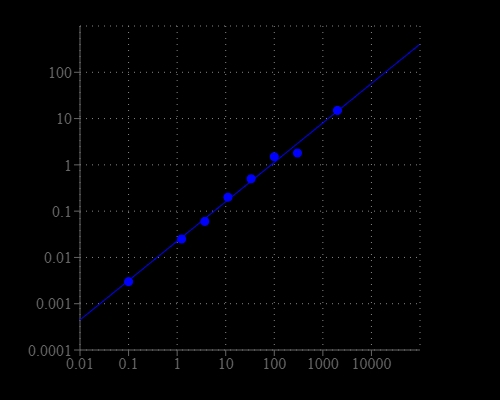
Acetylcholinesterase, also known as AChE, is an enzyme that degrades (through its hydrolytic activity) the neurotransmitter acetylcholine, producing choline and an acetate group. It is mainly found at neuromuscular junctions and cholinergic synapses in the central nervous system, where its activity serves to terminate synaptic transmission. AChE has a very high catalytic activity- each molecule of AChE degrades about 5000 molecules of acetylcholine per second. Acetylcholinesterase is also found on the red blood cell membranes, where it constitutes the Yt blood group antigen. Acetylcholinesterase exists in multiple molecular forms, which possess similar catalytic properties, but differ in their oligomeric assembly and mode of attachment to the cell surface. This Amplite® Colorimetric Acetylcholinesterase Assay Kit provides a convenient method for the detecting AChE activity. The kit uses DTNB to quantify the thiolcholine produced from the hydrolysis of acetylthiolcholine by AChE. The absoprtion intensity of DTNB adduct is proportional to the formation of thiolcholine, thus the AChE activity.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 11400 | 200 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Instrument settings
| Absorbance microplate reader | |
| Absorbance | 410 ± 5 nm |
| Recommended plate | Clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 20, 2026
